753009
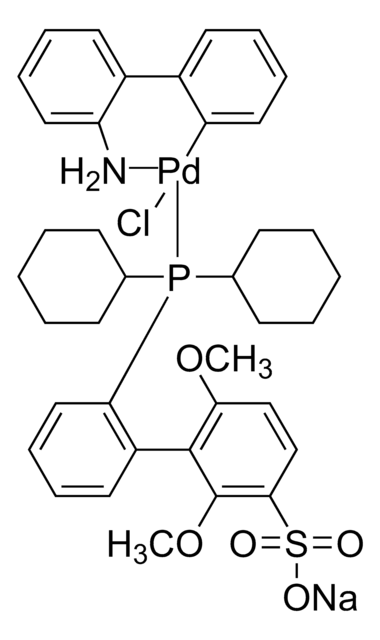
SPhos Pd G2
Sinónimos:
2nd Generation SPhos Precatalyst, Chloro(2-dicyclohexylphosphino-2′,6′-dimethoxy-1,1′-biphenyl)[2-(2′-amino-1,1′-biphenyl)]palladium(II), SPhos-Pd-G2
About This Item
Productos recomendados
form
solid
Quality Level
feature
generation 2
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
218 °C (dec.)
functional group
phosphine
SMILES string
COC1=CC=CC(OC)=C1C2=C(P(C3CCCCC3)C4CCCCC4)C=CC=C2.NC5=C(C6=C([Pd]Cl)C=CC=C6)C=CC=C5
InChI
1S/C26H35O2P.C12H10N.ClH.Pd/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;/h9-11,16-21H,3-8,12-15H2,1-2H3;1-6,8-9H,13H2;1H;/q;;;+1/p-1
InChI key
NOMWEFBAPVZIIP-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Self-arylation of 1-alkyl-3-bromo-2-phenyl-2,1-borazaronaphthalenes to form 2,1-borazaronaphthols.
- Suzuki–Miyaura coupling of [Ru(bpy)2]+ with 4-carboxyphenylboronic acid.
- Suzuki–Miyaura coupling of β-borylated porphyrins with 2-iodoaniline.
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Tools aid in kit setup for organic chemistry techniques, ensuring ease and success.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico