423602
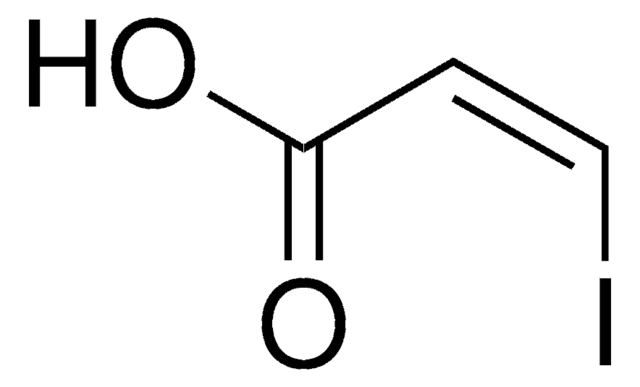
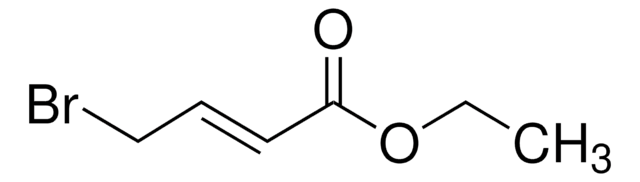
Ethyl cis-3-iodoacrylate
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
ICH=CHCO2C2H5
Número de CAS:
Peso molecular:
226.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.533 (lit.)
bp
85 °C/20 mmHg (lit.)
density
1.765 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(I)=C(/[H])C(=O)OCC
InChI
1S/C5H7IO2/c1-2-8-5(7)3-4-6/h3-4H,2H2,1H3/b4-3-
InChI key
AELYFQSZXFFNGP-ARJAWSKDSA-N
General description
Ethyl cis-3-iodoacrylate (cis-ethyl-β-iodoacrylate, ethyl (Z)-β-iodoacrylate) is Z-vinyl iodide derivative. It is reported to be prepared from ethyl propiolate by treating with sodium iodide. Its Suzuki coupling reaction with cyclic vinyl boronic acids has been studied.
Application
Ethyl cis-3-iodoacrylate may be used in the synthesis of the following:
- vinyl sulfides
- (Z)-1-iodohept-1-en-3-ol
- (E)-1-iodohept-1-en-3-ol
- (Z)-β-iodoacrolein
- 6-oxo-M1Guo
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
210.2 °F - closed cup
flash_point_c
99 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Stereo-and regiospecific Cu-catalyzed cross-coupling reaction of vinyl iodides and thiols: a very mild and general route for the synthesis of vinyl sulfides.
Kabir MS, et al.
Organic Letters, 10(15), 3363-3366 (2008)
Regiochemical control in the metal-catalyzed transposition of allylic silyl ethers.
Hansen EC and Lee D.
Journal of the American Chemical Society, 128(25), 8142-8143 (2006)
Selection of monoclonal antibodies against 6-oxo-M1dG and their use in an LC-MS/MS assay for the presence of 6-oxo-M1dG in vivo.
Akingbade D, et al.
Chemical Research in Toxicology, 25(2), 454-461 (2012)
A simple and convenient method for the preparation of (Z)-β-iodoacrolein and of (Z)- or (E)-γ-iodo allylic alcohols: (Z)- and (E)-1-iodohept-1-en-3-ol.
Marek I, et al.
Organic Syntheses, 74, 194-194 (1997)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico