262218
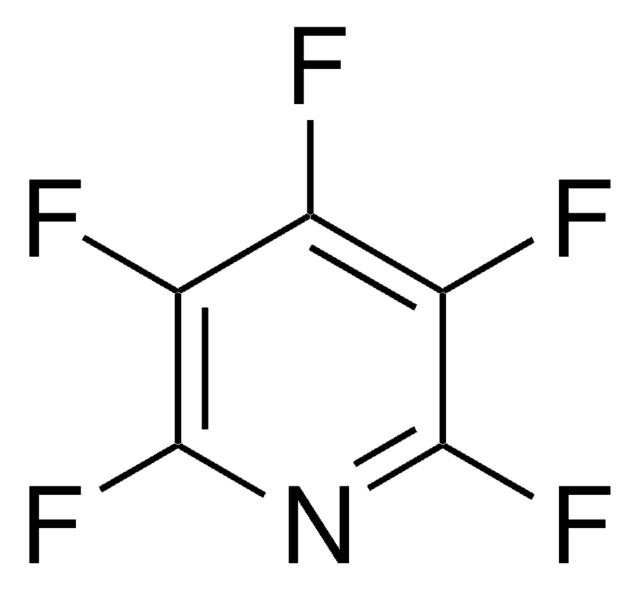
Pentafluoronitrobenzene
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6F5NO2
Número de CAS:
Peso molecular:
213.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
158-161 °C (lit.)
density
1.656 g/mL at 25 °C (lit.)
functional group
fluoro
nitro
SMILES string
[O-][N+](=O)c1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F5NO2/c7-1-2(8)4(10)6(12(13)14)5(11)3(1)9
InChI key
INUOFQAJCYUOJR-UHFFFAOYSA-N
General description
Electron attachment to pentafluoronitrobenzene has been studied in the energy range 0-16eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. The electroreduction of pentafluoronitrobenzene in dimethylformamide solution results in the formation of the dimer, octafluoro-4,4′-dinitro-biphenyl.
Application
Pentafluoronitrobenzene has been used in the preparation of p-azidotetrafluoroaniline, a new photoaffinity reagent.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
195.8 °F - closed cup
flash_point_c
91 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
K A Chehade et al.
The Journal of organic chemistry, 65(16), 4949-4953 (2000-08-24)
p-Azidotetrafluoroaniline (1) was synthesized in 65-73% yield by two different methods employing a stable carbamate intermediate. The first method trapped the intermediate isocyanate generated via a modified Curtius rearrangement with 2-methyl-2-propanol or 2-(trimethylsilyl)ethanol to form the stable carbamates 2d and
Judith Langer et al.
Physical chemistry chemical physics : PCCP, 10(11), 1523-1531 (2008-03-11)
Electron attachment to pentafluorobenzonitrile (C(6)F(5)CN) and pentafluoronitrobenzene (C(6)F(5)NO(2)) is studied in the energy range 0-16 eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. We find that pentafluoronitrobenzene exclusively generates fragment anions via
Voltammetry under high mass transport conditions. The application of the high speed channel electrode to the reduction of pentafluoronitrobenzene.
Coles BA, et al.
Journal of Electroanalytical Chemistry, 411(1), 121-127 (1996)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico