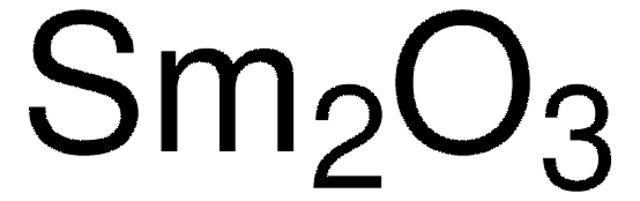228672
Samarium(III) oxide
99.9% trace metals basis
Sinónimos:
Samaria
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Sm2O3
Número de CAS:
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Productos recomendados
Quality Level
assay
99.9% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: samarium
density
8.35 g/mL at 25 °C (lit.)
SMILES string
O=[Sm]O[Sm]=O
InChI
1S/3O.2Sm
InChI key
PRCWVHVINXAFRG-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
S F Ahrabi et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 8(3), 193-201 (1999-06-24)
The effect of the neutron activation factors, i.e., admixture of samarium oxide (Sm2O3) and irradiation time, on the physico-chemical properties of the raw materials and the in vitro dissolution and disintegration of hydrophilic and lipophilic suppositories was investigated. It was
Lloyd G Tillman et al.
Journal of pharmaceutical sciences, 97(1), 225-236 (2007-08-28)
Treatment of systemic disease with phosphorothioate antisense oligonucleotides (PS ASOs) has been accomplished using local or parenteral routes of administration to date. This report describes, for the first time, the effective oral delivery of a second generation oligonucleotide where significant
Matthew D Burke et al.
Pharmaceutical research, 24(4), 695-704 (2007-03-21)
To develop a robust radiolabeling technique to enable evaluation of difficult to radiolabel gastric retentive formulations using gamma scintigraphy. The use of a successful radiolabel will allow accurate assessment of the gastric residence time of the formulations. The retention of
H M T U Herath et al.
Journal of biomedical materials research. Part A, 94(1), 130-136 (2010-02-04)
The biocompatibility of natural samarium (III) oxide, which has previously been used for treatment in bone-related diseases was determined as a first step in its evaluation as a bone implant material. Assessment for 28 days using osteoblast-like cells revealed no
Janne Marvola et al.
International journal of pharmaceutics, 281(1-2), 3-10 (2004-08-04)
This paper is a report from a pharmacoscintigraphic study with an Egalet constant-release system containing caffeine and natural abundance samarium oxide. First the formulation was tested in vitro to clarify integrity during irradiation in the nuclear reactor. Then six healthy
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico