220884
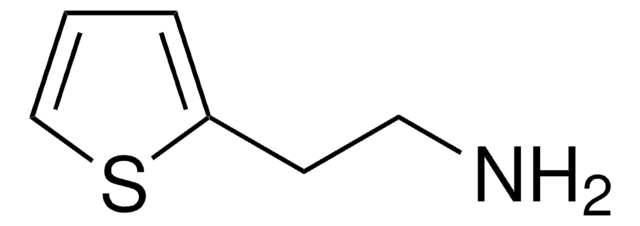
2-Thiophenemethylamine
96%
Sinónimos:
2-(Aminomethyl)thiophene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H7NS
Número de CAS:
Peso molecular:
113.18
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
96%
Formulario
liquid
liquid
índice de refracción
n20/D 1.5670 (lit.)
bp
95-99 °C/28 mmHg (lit.)
densidad
1.103 g/mL at 25 °C (lit.)
grupo funcional
amine
cadena SMILES
NCc1cccs1
InChI
1S/C5H7NS/c6-4-5-2-1-3-7-5/h1-3H,4,6H2
Clave InChI
FKKJJPMGAWGYPN-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
2-Thiophenemethylamine is a potential ligand replacement for poly(3-hexylthiophene)/CdSe hybrid solar cells.
Aplicación
2-Thiophenemethylamine was used in preparation of:
- naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one
- fluorescent Pd2+ sensor, N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide
Reactant involved in synthesis of:
Reactant involved in:
- Triazole-linked-thiopene conjugates for use as a biomimetic model for studies of metal detoxification and oxidative stress involving metallothionein
- Serotonin 5-HT1A receptor antagonists which have neuroprotective affects against ischemic cell damage
- Imidazole- and piperonyl-containing thiadiazoles and pyrimidines for use as inducible oxide synthase dimerization inhibitors
- Optoelectronic segmented polyurethanes
Reactant involved in:
- Studies of organocatalyzed asymmetric reductive amination of ketones
- Metal-free aerobic oxidative coupling of amines to imines
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Skin Corr. 1B
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
165.2 °F - closed cup
Punto de inflamabilidad (°C)
74 °C - closed cup
Equipo de protección personal
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Liping Duan et al.
Chemical communications (Cambridge, England), (47)(47), 6339-6341 (2008-12-03)
A new fluorescent Pd2+ sensor , N-butyl-4-(p-methyloxy)-phenylethynyl-5-thiophenemethylamino-1,8-naphthalimide, was designed and synthesized. It showed highly selective on-off fluorescence changes for Pd2+ among the representative transition and heavy metallic cations, and its fluorescence was efficiently quenched by 5 equivalents of Pd2+ in
Debasis Karak et al.
Dalton transactions (Cambridge, England : 2003), 42(19), 6708-6715 (2013-04-10)
A naphthalene-thiophene hybrid molecule (Z)-1-((thiophen-2-ylmethylamino)methylene)naphthalen-2(1H)-one () was prepared by condensation of 2-thiophenemethylamine and 2-hydroxy-1-naphthaldehyde. According to FTIR, (1)H NMR spectrometry and single crystal X-ray analysis, exists in the cis-keto-amine tautomeric form. behaves like a molecular AND type binary logic gate
Jun Yan Lek et al.
ACS applied materials & interfaces, 3(2), 287-292 (2011-01-26)
For hybrid solar cells, interfacial chemistry is one of the most critical factors for good device performance. We have demonstrated that the size of the surface ligands and the dispersion of nanoparticles in the solvent and in the polymer are
Adesola Abimbola Adeleke et al.
Journal of inorganic biochemistry, 214, 111266-111266 (2020-11-10)
Synthesis and spectroscopic characterization of five ligands ((E)-2-((pyridin-2-ylmethylene)amino)phenol L1, 2-(pyridin-2-yl)benzo[d]thiazole L2, (E)-N-(2-fluorophenyl)-1-(pyridin-2-yl)methanimine L3, (E)-1-(pyridin-2-yl)-N-(p-tolyl)methanimine L4 and (E)-1-(pyridin-2-yl)-N-(thiophen-2-ylmethyl)methanimine L5 along with fifteen silver(I) complexes of L1 - L5, with a general formula [AgL2]+X- (L = Schiff base and X = NO3-, ClO4- or CF3SO3-) is
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico