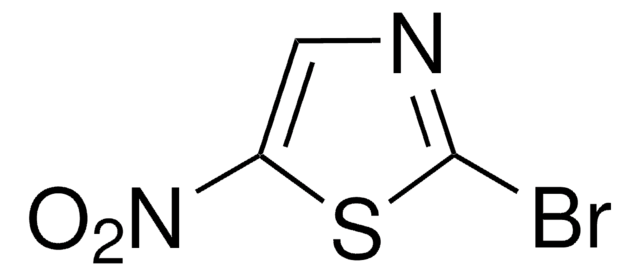
160474
2-Bromothiazole
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C3H2BrNS
Número de CAS:
Peso molecular:
164.02
Beilstein/REAXYS Number:
105724
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
liquid
refractive index
n20/D 1.593 (lit.)
bp
171 °C (lit.)
density
1.82 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
Brc1nccs1
InChI
1S/C3H2BrNS/c4-3-5-1-2-6-3/h1-2H
InChI key
RXNZFHIEDZEUQM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
2-Bromothiazole was used to N-arylate 5- and 7-azaindoles. 2-Bromothiazole was also used as starting reagent in the synthesis of:
- 2-cyanothiazole via cpper-catalyzed cyanation
- 2,4,5-trisubstituted thiazoles
- novel electron-deficient fused pyrrolo[3,2-d:4,5-d′]bisthiazole
- 3-(2′-thiazoyl)indoles
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
152.6 °F - closed cup
flash_point_c
67 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Synlett, 555-555 (2007)
Tetrahedron Letters, 48, 4831-4831 (2007)
Mohammed Al-Hashimi et al.
Organic letters, 12(23), 5478-5481 (2010-11-12)
The synthesis of a novel electron-deficient fused pyrrolo[3,2-d:4,5-d']bisthiazole is reported from 2-bromothiazole. This was copolymerized with thiophene, selenophene, thienothiophene, and bithiophene by microwave-assisted Stille polycondensation. The resulting polymers exhibited small optical band gaps combined with low-lying HOMO energy levels and
Synthesis of camalexin and related phytoalexins.
Ayer WA, et al.
Tetrahedron, 48(14), 2919-2924 (1992)
Cora Dunst et al.
The Journal of organic chemistry, 76(16), 6972-6978 (2011-07-09)
A general method for the synthesis of 2,4,5-trisubstituted thiazoles has been developed. Starting from commercially available 2-bromothiazole, successive metalations using TMPMgCl·LiCl or TMP(2)Zn·2MgCl(2)·2LiCl lead to the corresponding magnesated or zincated thiazoles which readily react with various electrophiles providing highly functionalized
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)