128562
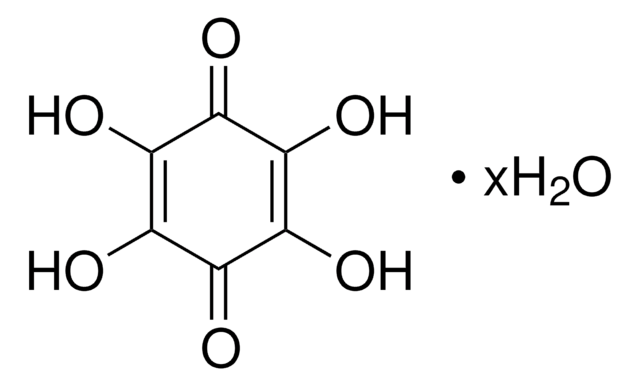
Hexaketocyclohexane octahydrate
97%
Sinónimos:
Cyclohexanehexone octahydrate, Triquinoyl octahydrate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6O6 · 8H2O
Número de CAS:
Peso molecular:
312.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
powder
mp
99 °C (dec.) (lit.)
functional group
ketone
storage temp.
2-8°C
SMILES string
[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].O=C1C(=O)C(=O)C(=O)C(=O)C1=O
InChI
1S/C6O6.8H2O/c7-1-2(8)4(10)6(12)5(11)3(1)9;;;;;;;;/h;8*1H2
InChI key
MQIMWEBORAIJPP-UHFFFAOYSA-N
General description
Hexaketocyclohexane octahydrate also known as Triquinoyl octahydrate is an organic compound, often utilized as a building block in various organic reactions, including the synthesis of water-soluble π-conjugated hexaazatrinaphthylenes and their derivatives.
Application
Hexaketocyclohexane octahydrate has been used in the preparation of hexaazatriphenylenehexacarbonitrile.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Synthesis and some reactions of hexaazatriphenylenehexanitrile, a hydrogen-free polyfunctional heterocycle with D3h symmetry.
Kanakarajan K and Czarnik AW.
The Journal of Organic Chemistry, 51(26), 5241-5243 (1986)
Hao Huang et al.
Advanced science (Weinheim, Baden-Wurttemberg, Germany), 7(9), 2000012-2000012 (2020-05-10)
The 2D conductive metal-organic frameworks (MOFs) are expected to be an ideal electrocatalyst due to their high utilization of metal atoms. Exploring a new conjugated ligand with extra active metallic center can further boost the structural advantages of conductive MOFs.
Lei Wang et al.
Nanoscale, 9(12), 4090-4096 (2017-03-16)
Nanostructured semiconducting polymers have emerged as a very promising class of metal-free photocatalytic materials for solar water splitting. However, they generally exhibit low efficiency and lack the ability to utilize long-wavelength photons in a photocatalytic oxygen evolution reaction (OER). Here
Nitrogen-rich layered carbon for adsorption of typical volatile organic compounds and low-temperature thermal regeneration
Li Tan, et al.
Journal of Hazardous Materials, 424, 127348-127348 (2022)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11-hexacarbonitrile 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/151/558/c0e2c95f-5228-4864-a7a5-4b9765a19840/640/c0e2c95f-5228-4864-a7a5-4b9765a19840.png)