112631
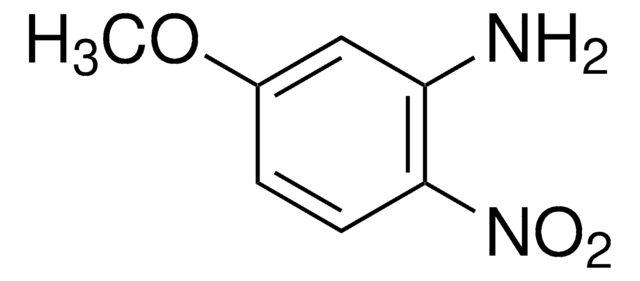
2-Methoxy-4-nitroaniline
98%
Sinónimos:
4-Nitro-o-anisidine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3OC6H3(NO2)NH2
Número de CAS:
Peso molecular:
168.15
Beilstein/REAXYS Number:
879619
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
mp
140-142 °C (lit.)
SMILES string
COc1cc(ccc1N)[N+]([O-])=O
InChI
1S/C7H8N2O3/c1-12-7-4-5(9(10)11)2-3-6(7)8/h2-4H,8H2,1H3
InChI key
GVBHRNIWBGTNQA-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
2-Methoxy-4-nitroaniline is an important inducer of CYP1A2 owing to its small molecular size.
Application
2-Methoxy-4-nitroaniline is used as a photometric reagent for the determination of ethinylestradiol (ETE), a semi-synthetic estrogen that is widely used in oral contraceptives.
Biochem/physiol Actions
The metabolism of 2-methoxy-4-nitroaniline (MNA) occurs via the hydroxylation of the phenyl ring to form 6-hydroxy MNA in Harlan Sprague Dawley rats and B6C3F(1)/N mice.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Use of nitroanilines for spectrophotometric determination of ethinylestradiol in pharmaceutical formulations.
Leonardo SG, et al.
Analytical Methods : Advancing Methods and Applications, 3(5), 1198-1201 (2011)
James M Mathews et al.
Xenobiotica; the fate of foreign compounds in biological systems, 42(12), 1213-1224 (2012-06-26)
The disposition of 2-Methoxy-4-nitroaniline (MNA) was investigated in male and female Harlan Sprague Dawley rats and B6C3F(1)/N mice following oral, intravenous, and dermal exposure to [(14)C]MNA at 2, 15, or 150 mg/kg. Clearance of MNA was investigated in male and
M Degawa et al.
Cancer letters, 96(1), 95-98 (1995-09-04)
Male F344 rats were treated with a chemical (aniline, nitrobenzene, 2-methoxy-p-phenylenediamine, 2-methoxy-4-nitroaniline or 2-methoxy-4-nitroazobenzene) produced by the azo-reduction and/or N-oxidation of 2-methoxy-4-amino-azo-benzene, a selective inducer of cytochrome P450IA2 (CYP1A2), and their effects on the induction of CYP1A enzymes in the
Rachel P Frawley et al.
Toxicology, 441, 152474-152474 (2020-05-08)
2-Methoxy-4-nitroaniline (MNA), an intermediate in the synthesis of azo dyes used in textiles and paints, is structurally similar to carcinogenic anilines. Human exposure occurs primarily in the occupational setting through handling of dye dust, and through use and disposal of
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico