109975
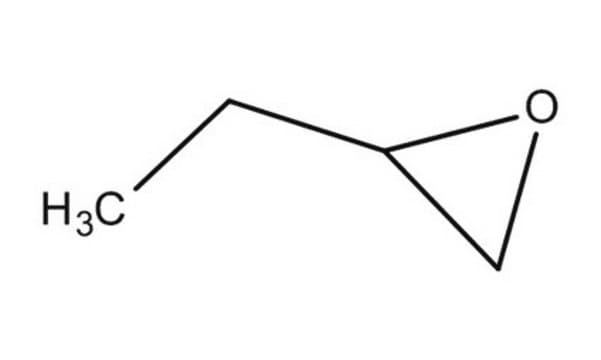
1,2-Epoxybutane
99%
Sinónimos:
α-Butylene oxide, 1,2-Butylene oxide, 1-Butene oxide, Ethyloxirane
About This Item
Productos recomendados
vapor density
2.2 (vs air)
Quality Level
vapor pressure
140 mmHg ( 20 °C)
assay
99%
autoignition temp.
698 °F
expl. lim.
19 %
refractive index
n20/D 1.384 (lit.)
bp
63 °C (lit.)
density
0.829 g/mL at 25 °C (lit.)
SMILES string
CCC1CO1
InChI
1S/C4H8O/c1-2-4-3-5-4/h4H,2-3H2,1H3
InChI key
RBACIKXCRWGCBB-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- As a monomer to synthesize novel initiators via ring-opening polymerization. These initiators can be used to prepare complex macromolecules such as grafted polyamides.
- To functionalize polyethyleneimine which is used in the synthesis of oxidation-stable adsorbents for CO2 capture.
Features and Benefits
- High polymerizability
- Low susceptibility to transfer reaction
- Ease of handling
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
5.0 °F - closed cup
flash_point_c
-15 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico