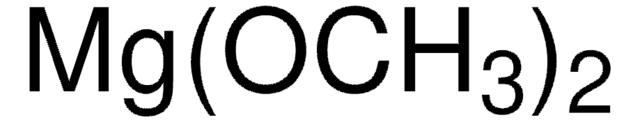292788
Potassium methoxide
95%
Synonym(s):
Potassium methylate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3OK
CAS Number:
Molecular Weight:
70.13
Beilstein:
3551544
EC Number:
MDL number:
UNSPSC Code:
12352107
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
SMILES string
[K+].C[O-]
InChI
1S/CH3O.K/c1-2;/h1H3;/q-1;+1
InChI key
BDAWXSQJJCIFIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Potassium methoxide is a basic nucleophile used in the dehalogenation of arylhalides.
Application
Potassium methoxide can be used:
- As a methoxylating agent for methoxylation of bromo- and mesyloxyalkanes in the presence of an ionic liquid [bmim][BF4].
- As a catalyst for the synthesis of dimethyl carbonate and methyl formate.
- As a catalyst for the transesterification of sunflower oil for the production of biodiesel.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Self-heat. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 2
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
General and practical potassium methoxide/disilane-mediated dehalogenative deuteration of (hetero) arylhalides
Xin W et al.
Journal of the American Chemical Society, 140, 10970-10974 (2018)
Synthesis of dimethyl carbonate from methanol and carbon dioxide using potassium methoxide as catalyst under mild conditions.
Cai Q, et al.
Catalysis Letters, 103(3-4), 225-228 (2005)
Significantly enhanced reactivities of the nucleophilic substitution reactions in ionic liquid.
Kim DW, et al.
The Journal of Organic Chemistry, 68(11), 4281-4285 (2003)
Integrated biodiesel production: a comparison of different homogeneous catalysts systems.
Vicente G, et al.
Bioresource Technology, 92(3), 297-305 (2004)
The effect of Mo(CO)6 as a co-catalyst in the carbonylation of methanol to methyl formate catalyzed by potassium methoxide under CO, syngas and H2 atmospheres. HP-IR observation of the methoxycarbonyl intermediate of Mo(CO)6.
Jali S, et al.
J. Mol. Catal. A: Chem., 348(1-2), 63-69 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service