LC-MS/MS Analysis of 16 PFAS in Salmon Using QuEChERS Based on FDA Method C-010.02
Per- and polyfluorinated alkyl substances (PFAS), also known as Forever Chemicals, are a class of compounds that have been widely used as commercial product applications over the past decades due to their versatile physical and chemical properties (e.g., water-repellent, firefighting foams, cookware, food packaging). Due to their chemical stability, PFAS chemicals are prevalent in our environment and pose a potential risk of bioaccumulation in humans over time. To limit the possible risks to human health, such as low infant birth weights, cancer, and impacts on the immune system, regulatory agencies such as EPA and FDA have established PFAS regulations encompassing limit values and PFAS analytical methods for specific PFAS substances.1-4 The U.S. Food and Drug Administration (FDA) issued advisories for PFAS extraction from food samples applying modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) methodology extraction and clean-up step (using dispersive solid phase extraction - dSPE), and an additional clean-up of the obtained QuEChERS extract using weak anion exchange (WAX) SPE columns, followed by LC-MS/MS analysis.4 For FDA method C-010.024, an extraction salt mixture containing 6.0 g MgSO4 as well as 1.5 g NaCl, and a dSPE clean-up mix containing 900 mg MgSO4, 300 mg primary secondary amine (PSA) and 150 mg graphitized carbon black, are specified. The Supel™ QuE non-buffered extraction salt mix (55295-U) and the newly developed Supel™ QuE PSA/ENVI-Carb™ Tube 3 for clean-up (55479-U) meet these method requirements. For further clean-up in this study, Supelclean™ ENVI-WAX™ SPE cartridges were used as a direct equivalent to the specified SPE cartridges in the FDA method C-010.02. This application note outlines one of the PFAS detection methods, developed in accordance with the FDA method C-010.02, for analyzing 16 PFAS compounds in salmon using LC-MS/MS.
Section Overview
Experimental— PFAS Analysis Method
Preparation of PFAS Standards and Solutions
Native and isotopically labeled PFAS standards were obtained as methanolic 50 µg/mL stock solutions. These standards were then diluted with methanol following the dilution scheme of the method C-010.02 to obtain the calibration standards in the required concentration range (external calibration: 0.01, 0.05, 0.10, 0.50, 1.0, 5.0, 10, and 25 ng/mL).
Sample Preparation
In accordance with U.S. FDA method C-010.02, PFAS analysis was performed for salmon samples. The salmon samples (5 g, homogenized with knife mill) were spiked at 0.05, 0.5 or 2.0 ng/g with 16 native PFAS as well as further spiked with 8 isotopically labeled internal standards at 2 ng/g. After adding 5 mL water, 150 µL formic acid and 10 mL acetonitrile, the samples were vigorously shaken for PFAS extraction. To test for potential PFAS background contamination, salmon samples were only spiked with isotopically labeled internal standards and equally processed. After addition of the Supel™ QuE salt package (6.0 g MgSO4, 1.5 g NaCl, 55295-U), the mixture was placed on a shaker for 10 minutes at 1500 rpm, followed by centrifugation for 10 minutes at 4000 g. The obtained supernatant was transferred into a second tube, the Supel™ QuE PSA/ ENVI-Carb™ Tube 3 (contains 900 mg MgSO4, 300 mg PSA, 150 mg ENVI-Carb™, 55479-U), and the sample was shaken for 10 minutes at 1500 rpm, followed by centrifugation for 10 minutes at 4000 g. Subsequently, the supernatants were filtered using Millex® Nylon 0.2 µm syringe filters (SLGNX13). For salmon samples, further SPE clean-up is required prior to LC-MS/MS analysis in accordance with the above FDA method. Therefore, 1 mL of the filtered supernatant was transferred into a tube and diluted to 15 mL with water. Supelclean™ ENVI-WAX™ SPE cartridges were used (500 mg, 6 mL, 54057-U) for the SPE clean-up, conditioned with 9 mL of 0.3% NH4OH in MeOH and equilibrated with 5 mL of water. After the salmon sample (15 mL) was loaded and passed through the cartridge, 5 mL of water was added as a washing step. The cartridge was subsequently dried for 1 min before 4 mL 0.3% NH4OH in MeOH was used to elute the analytes into an additional tube. Finally, the sample was evaporated to dryness, reconstituted in 1 mL MeOH and 5 µL of the internal standard N-EtFOSAA-d5 (200 ng/ml) were added prior to LC-MS/MS analysis.
LC-MS/MS analysis
An Agilent 1290 Infinity II instrument coupled to an Agilent 6495C triple quadrupole mass spectrometer was used for the LC-MS/MS analysis. Analyte separation was achieved using a Purospher® Star RP-18 endcapped (2 µm) Hibar HR (15 cm x 2.1 mm, 1.50649) as analytical column. In addition, a delay column (Purospher® Star RP18 endcapped Hibar RT (5 cm x 4.0 mm, 3 µm, 1.50428) was positioned after the mixing valve and before the autosampler to offset potential PFAS contamination originating from the LC system (e.g., pump, tubings, fittings, filters). The Purospher® STAR HPLC columns were chosen, as they generally provide stable HPLC separations even at higher pH, e.g. in the presence of basic mobile phase additives, such as 1-methyl piperidine recommended to be used in the C-010.02 method. We have tested the method with and without addition of the 1-methyl piperidine to the mobile phase. As no significant sensitivity gain was obtained using the additive in the presented application, however, the separation was performed without using 1-methyl piperidine, but the column was kept for this application to be prepared also for basic MP conditions. To avoid possible PFAS adsorption to the glass surface, polypropylene snap caps vials were used instead of standard glass vials. The LC and MS conditions are listed in Table 1.
Results and Discussion
A chromatogram of a solvent calibration standard containing the 16 native compounds is shown in Figure 1. The lower limit of quantitation (LLOQ) for all 16 compounds was 0.01 ng/mL for the LC-MS/MS method, and 0.02 ng/g in relation to the salmon sample. Linear calibration curves (0.01 ─25 ng/mL) with R2 ≥0.99 were obtained for all PFAS analytes except for PFDA with 0.98 (Table 2).
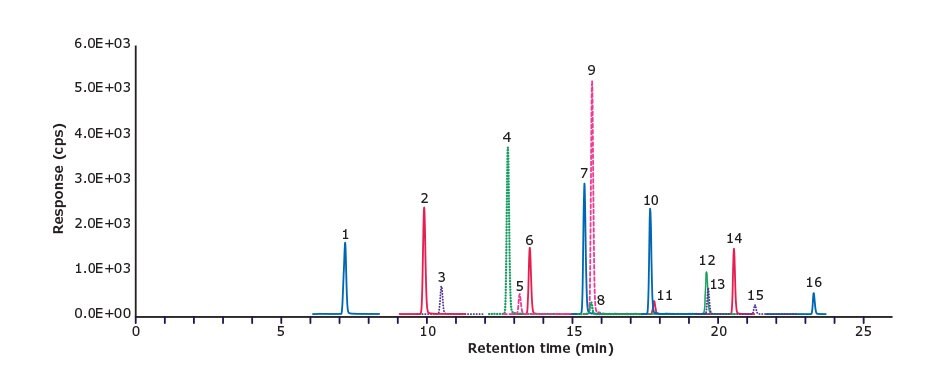
Figure 1.16 PFAS compounds at 1 ng/mL in methanol (Peak ID see Table 2)
The acceptable recovery range for the investigated PFAS analytes based on the FDA guidelines for the validation of chemical methods in food, feed, cosmetics and veterinary products is 40-120% (including RSD ≤ 22%) for concentrations at 0.001 mg/kg (1 ng/g). Table 3 displays the recoveries and %RSD from the experimental study where 16 compounds were spiked in triplicate to salmon samples at concentration levels of 0.05, 0.5, and 2.0 ng/g. The background evaluation of PFAS in the used salmon (salmon sample only spiked with isotopically labeled internal standards) revealed analyte concentrations for 3 PFAS analytes present above LLOQ (0.02 ng/g for PFBA and PFOS, 0.04 ng/g for PFOA). To accurately calculate the analyte recovery at the lowest spiking level (0.05 ng/g) for PFBA, PFOS and PFOA, the background levels found in salmon were subtracted accordingly. All obtained recoveries and %RSD were in the recommended range and thus meet the requirements of the FDA method.5
Conclusion
In this application note, the workflow of the FDA method C-010.02 for analysis of 16 PFAS in salmon samples based on a QuEChERS approach was investigated. At fortification levels of 0.05 ng/g, 0.5 ng/g and 2.0 ng/g, recoveries for all 16 compounds were within the acceptable range specified in the relevant FDA guideline for method validation. The calculated % RSDs were below 22%, further indicating a satisfactory precision. Hence, the employed combination of the new Supel™ QuE PSA/ENVI-Carb™ Tube 3 clean-up mix (55479-U), alongside the Supel™ QuE extraction salt mix, the Supelclean™ ENVI-WAX™ SPE cartridges, and Millex syringe filters, demonstrated efficacy as suitable sample preparation products in PFAS testing methods, for achieving the desired clean-up of food samples. The Purospher® STAR RP-18 HPLC columns were used in this method, as these generally provide stable separations in a wider range of mobile phase pH values, e.g. in the presence of basic mobile phases additives, such as 1-methyl piperidine described in the C-010.02 method.
PFAS Analytical Standards
Sample preparation, HPLC, and Accessories
See also our dedicated 2.7 µm Fused-Core® particle Ascentis® Express 90 Å PFAS and the Ascentis® Express 90 Å PFAS Delay columns.
Read more about PFAS testing at SigmaAldrich.com/pfas
REFERENCES
Para seguir leyendo, inicie sesión o cree una cuenta.
¿No tiene una cuenta?