1A00050
USP
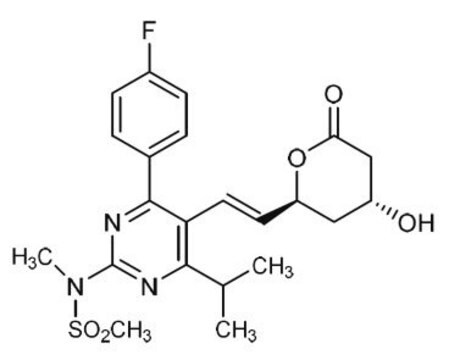
Rosuvastatin Ketone
Pharmaceutical Analytical Impurity (PAI)
Sinónimos:
(3R,6E)-7-[4-(4-Fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoic acid, (R,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)-3-hydroxy-5-oxohept-6-enoic acid, (3R,6E)-7-[4-(4-Fluorophenyl)-2-(N-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3-hydroxy-5-oxohept-6-enoic acid, 5-Oxorosuvastatin
About This Item
Productos recomendados
grade
pharmaceutical analytical impurity (PAI)
agency
USP
API family
rosuvastatin
manufacturer/tradename
USP
application(s)
pharmaceutical
format
neat
storage temp.
2-8°C
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Rosuvastatin Calcium
Therapeutic Area: Antihyperlipidemics
For more information about this PAI, visit here.
Application
Features and Benefits
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
Analysis Note
Other Notes
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
Haga su pedido de una amplia gama de materiales de referencia primarios muy caracterizados para utilizar con las monografías de la USP-NF pensadas para el análisis de sustancias farmacológicas y formas de administración, excipientes farmacéuticos, ingredientes y suplementos alimentarios.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Order from a broad range of highly characterized primary reference standard materials to use with USP-NF monographs for the testing of drug substances & dosage forms, pharmaceutical excipients, food ingredients and dietary supplements.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico