SML0055
Securinine
≥98% (HPLC)
About This Item
Productos recomendados
Nivel de calidad
Ensayo
≥98% (HPLC)
Formulario
powder
actividad óptica
[α]/D -980 to -1015 (C=1, MeOH)
color
yellow
solubilidad
DMSO: ≥25 mg/mL
temp. de almacenamiento
2-8°C
cadena SMILES
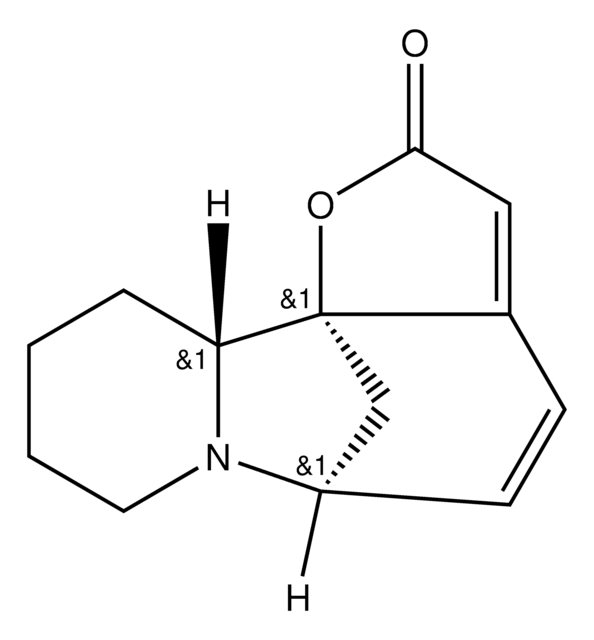
O=C1O[C@@]23C[C@@H](C=CC2=C1)N4CCCC[C@H]34
InChI
1S/C13H15NO2/c15-12-7-9-4-5-10-8-13(9,16-12)11-3-1-2-6-14(10)11/h4-5,7,10-11H,1-3,6,8H2/t10-,11-,13+/m1/s1
Clave InChI
SWZMSZQQJRKFBP-WZRBSPASSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Aplicación
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Oral
Código de clase de almacenamiento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico