M6760
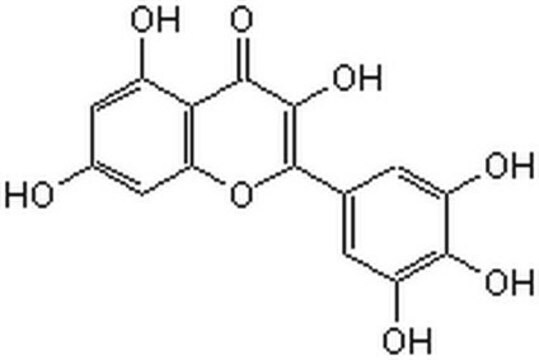
Myricetin
≥96.0%, crystalline
Sinónimos:
3,3′,4′,5,5′,7-Hexahydroxyflavone, Cannabiscetin, Myricetol
About This Item
Productos recomendados
assay
≥96.0%
form
crystalline
mp
>300 °C (lit.)
solubility
absolute ethanol: 10 mg/mL, clear to slightly hazy, yellow to very deep greenish-yellow
SMILES string
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
Inchi Key
IKMDFBPHZNJCSN-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- to investigate its effect on end product (AGE)- bovine serum albumin mediated phosphorylation of mitogen-activated protein kinase(ERK1)
- as a standard for the quantification of phenolics from noni plant extracts using high performance liquid chromatography(HPLC)
- as a standard for characterization of phenolic compounds from Hibiscus sabdariffa using ultra-high performance liquid chromatography(UHPLC)
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocolos
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico