G9753
α-D-Glucosamine 1-phosphate
Sinónimos:
2-Amino-2-deoxy-α-D-glucopyranosyl phosphate
About This Item
Productos recomendados
origen biológico
natural (inorganic)
Nivel de calidad
Ensayo
≥97% (TLC)
Formulario
powder
impurezas
<8.5% water (Karl Fischer)
color
white
solubilidad
water: 100 mg/mL, clear, colorless
temp. de almacenamiento
−20°C
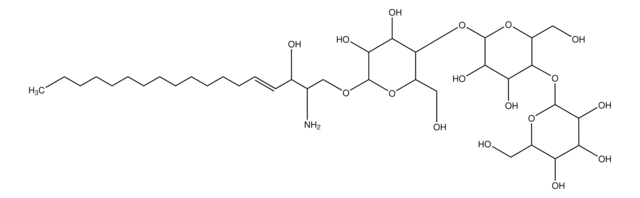
cadena SMILES
NC1C(O)C(O)C(CO)OC1OP(O)(O)=O
InChI
1S/C6H14NO8P/c7-3-5(10)4(9)2(1-8)14-6(3)15-16(11,12)13/h2-6,8-10H,1,7H2,(H2,11,12,13)
Clave InChI
YMJBYRVFGYXULK-UHFFFAOYSA-N
Categorías relacionadas
Aplicación
- Cellodextrin phosphorylase from Ruminiclostridium thermocellum: X-ray crystal structure and substrate specificity analysis. This study presents the enzymatic synthesis and analysis of alpha-ᴅ-Glucosamine 1-phosphate based polysaccharides using cellodextrin phosphorylase, showcasing potential for novel biomaterial development. Field et al., 2017
- Glucose-1-phosphate uridylyltransferase from Erwinia amylovora: Activity, structure and substrate specificity. This paper explores the biochemical pathway involving alpha-ᴅ-Glucosamine 1-phosphate in the context of bacterial metabolism, providing insights into microbial biochemistry and potential targets for antibacterial therapy. Field et al., 2017
Otras notas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico