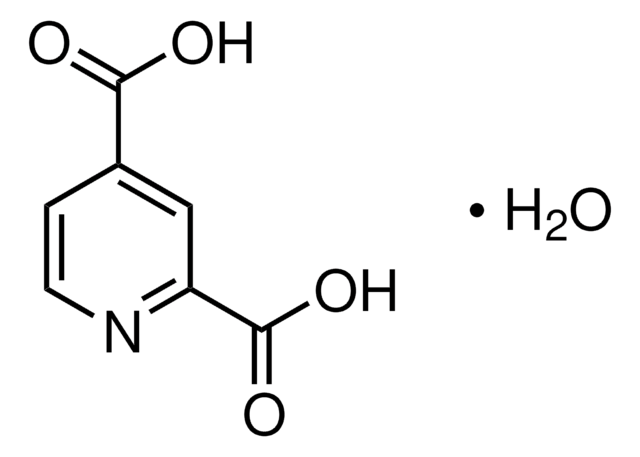
04473
2,4-Pyridinedicarboxylic acid
≥98.0%
Sinónimos:
Lutidinic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C7H5NO4
Número de CAS:
Peso molecular:
167.12
Beilstein:
131631
Número CE:
Número MDL:
Código UNSPSC:
12352106
ID de la sustancia en PubChem:
NACRES:
NA.25
Productos recomendados
Ensayo
≥98.0%
98.0-102.0% (T)
cadena SMILES
OC(=O)c1ccnc(c1)C(O)=O
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
Clave InChI
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Aplicación
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Rajarshi Choudhury et al.
FEBS letters, 581(14), 2733-2736 (2007-05-29)
Irrespective of their pyridine nucleotide specificity, all glutamate dehydrogenases share a common chemical mechanism that involves an enzyme bound 'iminoglutarate' intermediate. Three compounds, structurally related to this intermediate, were tested for the inhibition of purified NADP-glutamate dehydrogenases from two Aspergilli
Sue E Hutchinson et al.
Journal of biomolecular screening, 17(1), 39-48 (2011-08-24)
A high-throughput RapidFire mass spectrometry assay is described for the JMJD2 family of Fe(2+), O(2), and α-ketoglutarate-dependent histone lysine demethylases. The assay employs a short amino acid peptide substrate, corresponding to the first 15 amino acid residues of histone H3
Line H Kristensen et al.
The FEBS journal, 279(11), 1905-1914 (2012-03-17)
Dynamic methylations and demethylations of histone lysine residues are important for gene regulation and are facilitated by histone methyltransferases and histone demethylases (HDMs). KDM5B/Jarid1B/PLU1 is an H3K4me3/me2-specific lysine demethylase belonging to the JmjC domain-containing family of histone demethylases (JHDMs). Several
Florina Vlad et al.
Physiologia plantarum, 140(2), 199-207 (2010-06-18)
Prolyl 4-hydroxylases (P4Hs) catalyze the proline hydroxylation, a major post-translational modification, of hydroxyproline-rich glycoproteins. Two carnation petal P4H cDNAs, (Dianthus caryophyllus prolyl 4-hydroxylase) DcP4H1 and DcP4H2, were identified and characterized at the gene expression and biochemical level in order to
G Tschank et al.
The Biochemical journal, 275 ( Pt 2), 469-476 (1991-04-15)
The biochemical and morphological consequences of procollagen prolyl 4-hydroxylase inhibition by pyridine-2,4-dicarboxylic acid (2,4-PDCA) and its diethyl ester (diethyl-2,4-PDC) were studied in chick-embryo calvaria, which predominantly synthesize type I collagen. Half-maximal inhibition of tissue hydroxyproline formation required 650 microM-2,4-PDCA, whereas
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico