MAB1012
Anti-Alkaline Phosphatase Antibody, E. coli, bacterial only
ascites fluid, Chemicon®
Sinónimos:
AP, Alk. Phos.
About This Item
Productos recomendados
biological source
mouse
Quality Level
antibody form
ascites fluid
antibody product type
primary antibodies
clone
monoclonal
species reactivity
E. coli
manufacturer/tradename
Chemicon®
technique(s)
immunocytochemistry: suitable
immunoprecipitation (IP): suitable
western blot: suitable
isotype
IgG2a
UniProt accession no.
shipped in
dry ice
target post-translational modification
unmodified
Gene Information
Escherichia coli ... PhoA(945041)
Specificity
Immunogen
Application
Immunocytochemistry: reacts with E.coli. AP fusion protein targets in acetone fixed cell preparations. 1:4000, other fixatives or conditions untested.
ASSAY:
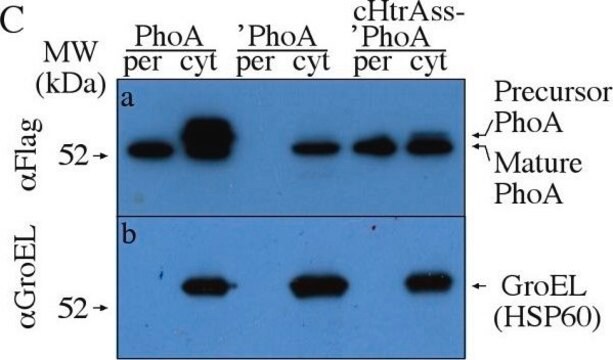
Preparation of E. coli TnphoA transformants: E. coli strain CC118 was transformed with plasmid pGEM-3Z containing TnphoA insertional mutations in the p101 gene of Mycoplasma hyorhinis, which encodes a protein with a typical N-terminal prokaryotic single peptide (Yogev et al. 1991).
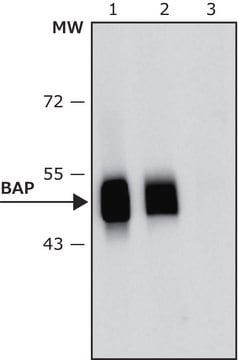
Identification of fusion protein with MAB1012 transformants: Transformants are grown in 2XYT medium to OD600=0.6. Cells were centrifuged 3 minutes at 10,000 x g, suspended in SDS-PAGE sample buffer, heated at 100°C for 5 minutes, frozen and thawed and centrifuged as above at room temperature to remove insoluble material. The sample is applied at 9% to a SDS-PAGE gel, and Western immunoblot is performed as described (Yogev et al. 1991).
Immunoprecipitation: 5μL of antibody per 500μL of lysate in RIPA or 0.5% triton X-100 solutions.
Optimal working dilutions must be determined by end user.
Physical form
Legal Information
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico