393204
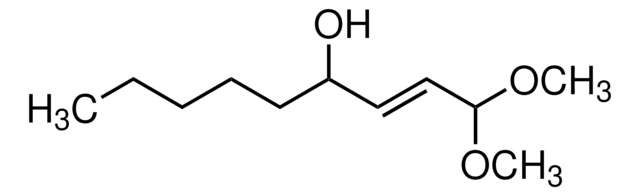
4-Hydroxynonenal
≥98% (HPLC), liquid, lipid peroxidation marker, Calbiochem®
Sinónimos:
4-Hydroxynonenal
About This Item
Productos recomendados
product name
4-Hydroxynonenal, 4-Hydroxynonenal, CAS 75899-68-2, is a major aldehyde product formed by peroxidation of ω-6-unsaturated fatty acids that is regarded as a specific marker of lipid peroxidation.
Quality Level
assay
≥98% (HPLC)
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
solubility
ethanol: 10 mg/mL
storage temp.
−70°C
InChI
1S/C9H16O2/c1-2-3-4-6-9(11)7-5-8-10/h5,7-9,11H,2-4,6H2,1H3/b7-5+
InChI key
JVJFIQYAHPMBBX-FNORWQNLSA-N
General description
Biochem/physiol Actions
Na+, K+-ATPase activity
Warning
Physical form
Reconstitution
Other Notes
Carini, R., et al. 1996. Biochem. Biophys. Res. Commun.218, 772.
Li, L., et al. 1996. Toxicol.Appl. Pharmacol.139, 135.
Siems, W.G., et al. 1996. Free Radic. Res.20, 215.
Ullrich, O., et al. 1996. Free Radic. Res.24, 421.
van Kuijk, F.J., et al. 1995. Anal. Biochem. 224, 420.
Esterbauer, H., et al. 1991. Free Radic. Biol. Med.11, 81.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
57.2 °F - closed cup
flash_point_c
14 °C - closed cup
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico