939412
n-Butil-litio solution

2.5 M (in PAO/hexanes mixture)
Sinónimos:
n-BuLi, Butil litio, Butil-litio solution, Litio-1-butanida
About This Item
Productos recomendados
form
liquid
Quality Level
concentration
2.5 M (in PAO/hexanes mixture)
color
colorless to yellow
storage temp.
2-8°C
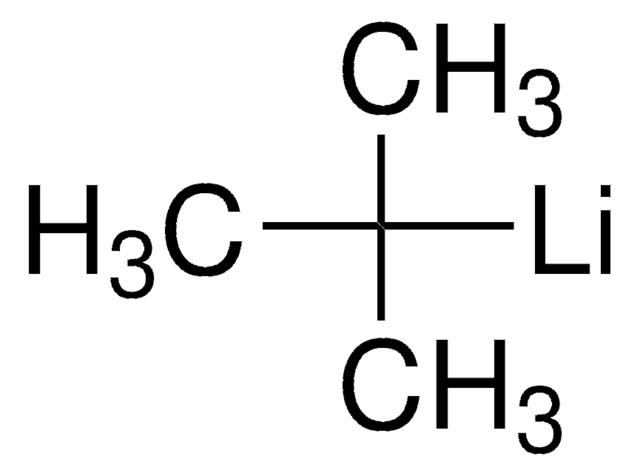
SMILES string
[Li]CCCC
InChI
1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
InChI key
MZRVEZGGRBJDDB-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
The product is also used in the following reactions:
- Anionic rearrangement reactions
- Metal-halogen interchange and transmetalation reactions
- Elimination reactions
- [1,2]- and [1,4]-Wittig rearrangement reaction
- Anionic homo-Fries rearrangement reaction
- Asymmetric carbolithiation
Features and Benefits
related product
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 3 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT RE 2 Inhalation - Water-react 1
target_organs
Nervous system
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
88.7 °F
flash_point_c
31.5 °C
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico