930083
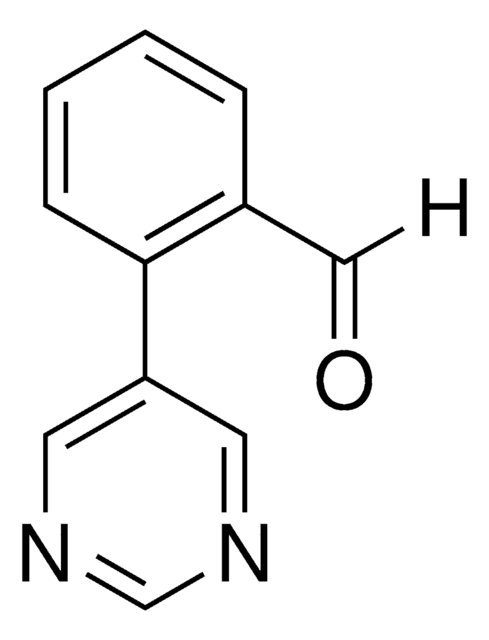
2-(Pyrimidin-5-yl)phenol
≥95%
Sinónimos:
Maiti-Bag-Dutta Auxiliary
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H8N2O
Número de CAS:
Peso molecular:
172.18
Número MDL:
Código UNSPSC:
12352100
NACRES:
NA.21
Productos recomendados
Aplicación
2-(Pyrimidin-5-yl)phenol is a temporary directing group (TDG) to assist as a co-catalyst for metal catalyzed C-H functionalization. Often in C-H functionalization, an auxiliary compound is used to control site selectivity. These traditionally are covalently bonded to the compound of interest, and must subsequently be removed after functionalization, like a typical protecting group. To simplify the process of C-H functionalization, 2-Fluoro-6-(pyrimidin-5-yl)aniline is one of a series of temporary directing groups developed by Deb Maiti′s lab that promote site selectivity without the inclusion of additional synthetic steps.
2-(Pyrimidin-5-yl)phenol is an effective TDG for meta directed C-H functionalization of substitutes with a variety of electron withdrawing functional groups, with high selectivity.
2-(Pyrimidin-5-yl)phenol is an effective TDG for meta directed C-H functionalization of substitutes with a variety of electron withdrawing functional groups, with high selectivity.
Producto relacionado
Referencia del producto
Descripción
Precios
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Sukdev Bag et al.
Journal of the American Chemical Society, 142(28), 12453-12466 (2020-06-05)
Controlling remote selectivity and delivering novel functionalities at distal positions in arenes are an important endeavor in contemporary organic synthesis. In this vein, template engineering and mechanistic understanding of new functionalization strategies are essential for enhancing the scope of such
Uttam Dutta et al.
Science (New York, N.Y.), 372(6543) (2021-05-15)
Transition metal-catalyzed aryl C-H activation is a powerful synthetic tool as it offers step and atom-economical routes to site-selective functionalization. Compared with proximal ortho-C-H activation, distal (meta- and/or para-) C-H activation remains more challenging due to the inaccessibility of these
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico