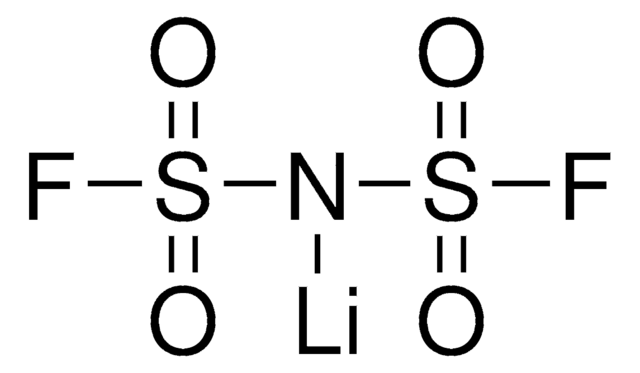919977
Bis(trifluorometano)sulfonimida lithium salt
anhydrous, 99.99% trace metals basis
Sinónimos:
Bis(trifluorometilsulfonil)amina lithium salt, Bistrifluorometanosulfonimidato de litio
About This Item
Productos recomendados
grado
anhydrous
Nivel de calidad
Ensayo
99.99% trace metals basis
características de los productos alternativos más sostenibles
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
234-238 °C (lit.)
aplicaciones
battery manufacturing
categoría alternativa más sostenible
cadena SMILES
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
Clave InChI
QSZMZKBZAYQGRS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
- High electrochemical stability
- High lithium-ion conductivity
- Thermal stability
- Hydrophilic nature
Aplicación
- An additive in the development of dual-functional separator coating materials. These materials are based on covalent organic frameworks (COFs) and are specifically designed for use in high-performance lithium-selenium sulfide batteries. The Li-SeS2 battery achieved outstanding performance in terms of energy storage and stability. It exhibited a specific capacity of 844.6 mA h g-1 at 0.5C and a SeS2 loading of 2 mg cm-2.
- As an additive in the electrolyte formulation along with polyethylene oxide for the development of solid-state lithium batteries. LiTFSI enhance the ionic conductivity of the PEO-based electrolyte, which is essential for the efficient transport of lithium ions.
- As a key component in the development of a PEO/LiTFSI-coated polypropylene membrane. This membrane is designed for high-loading lithium–sulfur batteries to enhance battery performance, improve capacity, and extend cycle life.
- As a component in the electrolyte system along with TEMPOL derivatives. The incorporation of LiTFSI in the electrolyte system enhances the stability and achieves an efficiency of 6.16% in solid-state fiber dye-sensitized solar cells.
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
Órganos de actuación
Nervous system
Código de clase de almacenamiento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico