The solubility of this product in CCL4 as well as the capability of replacing AIBN in the bromination reaction, has not been determined. It may be helpful to review the following literature:
check the chemical literature on this compound through (for example) PubChem: https://pubchem.ncbi.nlm.nih.gov/compound/1_1_-Azobis_cyclohexanecarbonitrile
380210
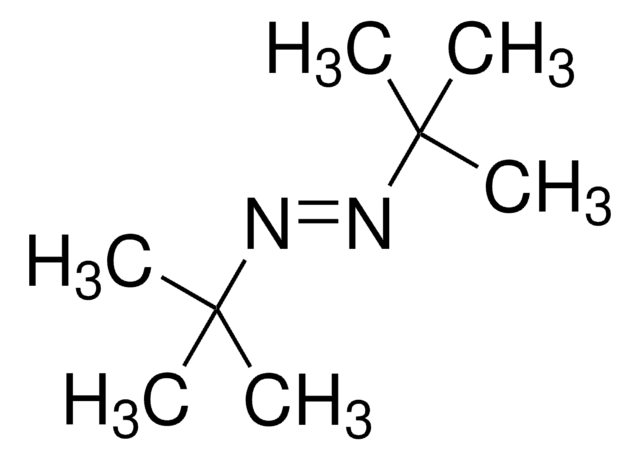
1,1′-Azobis(cyclohexanecarbonitrile)
98%
Sinónimos:
1,1′-Azobis(cyanocyclohexane), ACHN
About This Item
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
solid
mp
114-118 °C (lit.)
temp. de almacenamiento
2-8°C
cadena SMILES
N#CC1(CCCCC1)\N=N\C2(CCCCC2)C#N
InChI
1S/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2/b18-17+
Clave InChI
KYIKRXIYLAGAKQ-ISLYRVAYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Self-react. D - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
5.2 - Organic peroxides and self-reacting hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
Protocolos
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
-
Is ACHN sufficiently soluble in CCl4? Specifically, can it replace AIBN in the bromination reaction of 4'-(p-Tolyl)-2,2':6',2''-terpyridine with N-Bromosuccinimide? According to Inorg Chem 2003 42, 2908-2918.
1 answer-
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico