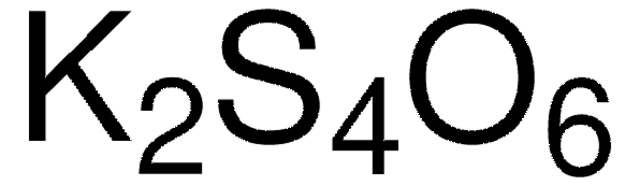S5758
Sodium tetrathionate dihydrate
≥98% (titration)
About This Item
Produits recommandés
Pureté
≥98% (titration)
Solubilité
H2O: 200 + 4mL H2O mg, clear to hazy, colorless to faintly yellow
Densité
2.1 g/mL at 25 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
O.O.[Na+].[Na+].[O-]S(=O)(=O)SSS([O-])(=O)=O
InChI
1S/2Na.H2O6S4.2H2O/c;;1-9(2,3)7-8-10(4,5)6;;/h;;(H,1,2,3)(H,4,5,6);2*1H2/q2*+1;;;/p-2
Clé InChI
HAEPBEMBOAIUPN-UHFFFAOYSA-L
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Gaussia princeps luciferase: a bioluminescent substrate for oxidative protein folding.: Sodium tetrathionate dihydrate is used to study its impact on the oxidative folding of proteins, utilizing Gaussia princeps luciferase as a bioluminescent substrate. This study advances understanding of protein chemistry and bioluminescence applications in biochemical assays (Yu T et al., 2018).
- A spectroscopic investigation into the reaction of sodium tetrathionate with cysteine.: The research utilizes spectroscopic methods to study the reaction between sodium tetrathionate dihydrate and cysteine, providing insights into the chemical interactions and potential applications in biochemistry and molecular biology (Church JS et al., 2008).
https://doi.org/10.2174/092986612800190973
https://doi.org/10.1080/10826068.2011.544230
https://doi.org/10.11150/kansenshogakuzasshi.83.380
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique