C8696
Cathepsin D from human liver
lyophilized powder, ≥250 units/mg protein (E1%/280)
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro CAS:
Numéro MDL:
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.32
Produits recommandés
Forme
lyophilized powder
Niveau de qualité
Activité spécifique
≥250 units/mg protein (E1%/280)
Poids mol.
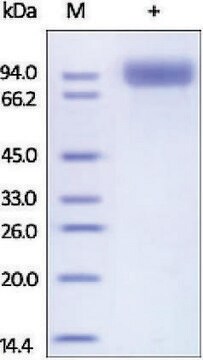
~45 kDa
Couleur
white
Numéro d'accès UniProt
Température de stockage
−20°C
Informations sur le gène
human ... CTSD(1509)
Description générale
Cathepsin D is an aspartic protease, which is located in lysosomes. It is involved in protein catabolism and maintains hormone and antigen processing. Cathepsin D is implicated in neoplasia and neurodegenerative changes. It regulates lysosomal proteolysis and endogenous fibrinolysis.
Application
Cathepsin D from human liver has been used:
- in β-secretase activity assay
- for enzymatic degradation of amyloid β 1-42
- in microinjection of human foreskin fibroblasts
Actions biochimiques/physiologiques
Cathepsin D is an endosomal-lysosomal aspartic protease implicated in breast cancer metastasis and Alzheimer′s disease. Lysosomal release of cathepsin D has been found to precede cytochrome c release and loss of membrane potential in apoptotic human foreskin fibroblasts. Cathepsin D levels in PC12 cells increase 12 to 24 hours after apoptosis is induced.
Autres remarques
Contains α, β and γ isoenzymes as observed by isoelectric-focusing.
Définition de l'unité
One unit will produce an increase in A280 of 1.0 in 30 min at pH 3.3 at 37 °C measured as TCA-soluble products using acid-denatured hemoglobin as substrate (1 cm light path).
Forme physique
Powder containing sodium phosphate buffer salt.
Inhibiteur
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Aspartic Proteinases Physiology and Pathology (1995)
Platelet membrane beta-secretase activity in mild cognitive impairment and conversion to dementia: a longitudinal study
McGuinness B, et al.
Journal of Alzheimer'S Disease, 49(4), 1095-1103 (2016)
Jeane do Nascimento Moraes et al.
The journal of venomous animals and toxins including tropical diseases, 28, e20220002-e20220002 (2022-11-22)
Cathepsin D (CatD) is a lysosomal proteolytic enzyme expressed in almost all tissues and organs. This protease is a multifunctional enzyme responsible for essential biological processes such as cell cycle regulation, differentiation, migration, tissue remodeling, neuronal growth, ovulation, and apoptosis.
Mengjie Ma et al.
Molecules (Basel, Switzerland), 28(13) (2023-07-14)
In this study, cathepsin D was oxidized in vitro with different concentrations of H2O2, and the activity, structure, and extent of myofibrillar protein degradation by oxidized cathepsin D were evaluated. The sulfhydryl content of cathepsin D decreased to 9.20% after
Marie Maynadier et al.
Journal of controlled release : official journal of the Controlled Release Society, 171(2), 251-257 (2013-08-01)
Implication of the intracellular proteolytic activity of Cathepsin D (CathD), a lysosomal aspartyl-protease overexpressed in numerous solid tumors, has been evidenced on tumor growth. Its intracellular inhibition by potent inhibitors such as pepstatin constitutes a relevant but challenging molecular target.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique