PHR1934
Ibuprofen Impurity B
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
Ibuprofen Impurity B Sodium Salt, (2RS)-2- (4-BUTYLPHENYL)PROPANOIC ACID SODIUM SALT
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
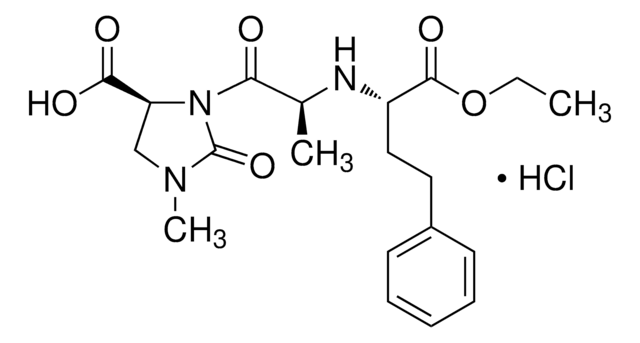
Formule empirique (notation de Hill):
C13H17O2Na
Numéro CAS:
Poids moléculaire :
228.26
Code UNSPSC :
41116107
Nomenclature NACRES :
NA.24
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to Ph. Eur. B1220000
Famille d'API
ibuprofen
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
pkg of 20 mg
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
Description générale
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the nonsteroidal anti-inflammatory drug (NSAID)― ibuprofen, used for the treatment of mild and moderate pain such as rheumatoid arthritis, osteoarthritis, and dysmenorrhea.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the nonsteroidal anti-inflammatory drug (NSAID)― ibuprofen, used for the treatment of mild and moderate pain such as rheumatoid arthritis, osteoarthritis, and dysmenorrhea.
Application
This pharmaceutical secondary standard can also be used as follows:
- Development of a reverse-phase ultraperformance liquid chromatographic (RP-UPLC) method for the estimation of ibuprofen and diphenhydramine citrate along with their related impurities in their combined dosage form
- Simultaneous determination of ibuprofen and its 17 related impurities by an ICH validated reversed-phase high-performance liquid chromatography (RP-HPLC) method in tablets
Remarque sur l'analyse
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Note de bas de page
To see an example of a Certificate of Analysis for this material enter LRAB8256 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
Produit(s) apparenté(s)
Réf. du produit
Description
Tarif
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Choose from one of the most recent versions:
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Development and validation of an HPLC method for simultaneous determination of ibuprofen and 17 related compounds
Han Z, et al.
Chromatographia, 80, 1353-1360 (2017)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique








![2-[4-(1-Hydroxy-2-methylpropyl)phenyl]propanoic acid pharmaceutical impurity standard](/deepweb/assets/sigmaaldrich/product/structures/194/635/6124adcb-841c-49b5-bfa2-91cacafbf2c6/640/6124adcb-841c-49b5-bfa2-91cacafbf2c6.png)