SYX00184
4-Hydrazino-1H-pyrimidin-2-one
AldrichCPR
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
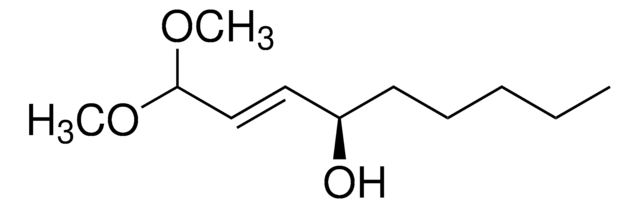
C4H6N4O
Numéro CAS:
Poids moléculaire :
126.12
Numéro MDL:
Code UNSPSC :
12352200
ID de substance PubChem :
Produits recommandés
Forme
solid
Chaîne SMILES
NNC1=NC(=O)NC=C1
InChI
1S/C4H6N4O/c5-8-3-1-2-6-4(9)7-3/h1-2H,5H2,(H2,6,7,8,9)
Clé InChI
ZMYMWRORNQVELJ-UHFFFAOYSA-N
Autres remarques
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Informations légales
Product of Synthonix
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Lot/Batch Number
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
M Aida et al.
Biochemical and biophysical research communications, 153(2), 552-557 (1988-06-16)
The intrinsic properties of N4-aminocytosine, a base analogue of cytosine, are analyzed by an ab initio molecular orbital method. Relative stabilities of four possible isomeric structures of N4-aminocytosine are shown. The more stable isomer has the smaller dipole moment, so
Synthesis and properties of oligodeoxyribonucleotides containing a mutagenic base, N4-aminocytosine.
S Kuwazura et al.
Nucleic acids symposium series, (27)(27), 117-118 (1992-01-01)
Oligodeoxyribonucleotides containing a mutagenic base analog, N4-aminocytosine, 5'-AATTGC(am)AATT-3' and 5'-AATTAC(am)AATT-3' (C(am); N4-aminocytosine) were prepared by chemical modification of 5'-AATTGCAATT-3' and 5'-AATTACAATT-3', respectively. The values of Tm were 29 degrees C for 5'-AATTGC(am)AATT-3' and 32 degrees C for 5'-AATTGCAATT-3'. In contrast
N Nitta et al.
Nucleic acids symposium series, (6)(6), s43-s44 (1979-01-01)
N4-Aminocytosine reacted with acetone and acetaldehyde to form hydrazones that were readily revertible to the parent compound. With pyruvate, in contrast, it formed a stable hydrazone. By use of bromopyruvate, N4-aminocytosine was linked to glutathione.
K Matsumoto et al.
Mutation research, 268(1), 59-64 (1992-07-01)
N4-Aminocytidine is mutagenic in various organisms. In the cell, this cytidine analog is metabolized into N4-aminodeoxycytidine 5'-triphosphate, which will then be incorporated into DNA and mutation will result during the replication of the DNA. To prove that the N4-aminocytosine residue
A Nomura et al.
Mutation research, 177(2), 283-287 (1987-04-01)
N4-Aminocytidine induced mutation to 6-thioguanine resistance in Chinese hamster lung V79 cells in culture. Previous studies with experimental systems of in vitro DNA synthesis and of phage and bacterial mutagenesis have shown that this nucleoside analog induces base-pair transitions through
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique