S5006
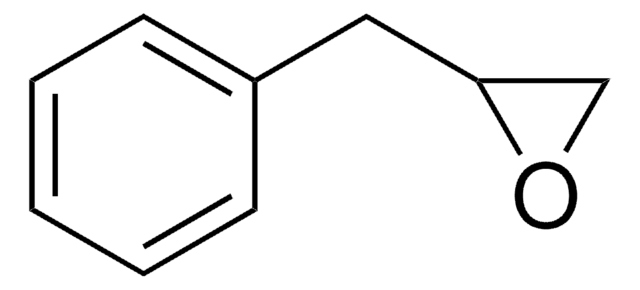
Styrene oxide
97%
Synonyme(s) :
1,2-Epoxyethylbenzene, Phenylethylene oxide, Phenyloxirane
About This Item
Produits recommandés
Densité de vapeur
4.14 (vs air)
Niveau de qualité
Pression de vapeur
<1 mmHg ( 20 °C)
Pureté
97%
Température d'inflammation spontanée
928 °F
Limite d'explosivité
~22 %
Indice de réfraction
n20/D 1.535 (lit.)
Point d'ébullition
194 °C (lit.)
Pf
−37 °C (lit.)
Densité
1.054 g/mL at 25 °C (lit.)
Chaîne SMILES
C1OC1c2ccccc2
InChI
1S/C8H8O/c1-2-4-7(5-3-1)8-6-9-8/h1-5,8H,6H2
Clé InChI
AWMVMTVKBNGEAK-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Synthesis of poly (styrene oxide) with different molecular weights using tin catalysts: This study explores the ring-opening polymerization of styrene oxide using tin catalysts to produce homopolymers with varying molecular weights (Kayan, 2015).
- Electrogenerated BF3 from tetrafluoroborate-based ionic liquids: theoretical and experimental studies towards selective styrene oxide isomerization: Research on using electrogenerated BF3 to selectively isomerize styrene oxide, highlighting theoretical and experimental insights (Bortolami et al., 2021).
- Selective conversion of styrene oxide to 2-phenylethanol in cascade reactions over non-noble metal catalysts: This paper investigates the catalytic conversion of styrene oxide to 2-phenylethanol using non-noble metal catalysts (Sasu et al., 2016).
- Laboratory blueprints for interstellar searches of aromatic chiral molecules: rotational signatures of styrene oxide: Study of the rotational spectra of styrene oxide for potential detection in interstellar space (Stahl et al., 2020).
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2 - Skin Sens. 1
Code de la classe de stockage
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
176.0 °F - closed cup
Point d'éclair (°C)
80 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique