745979
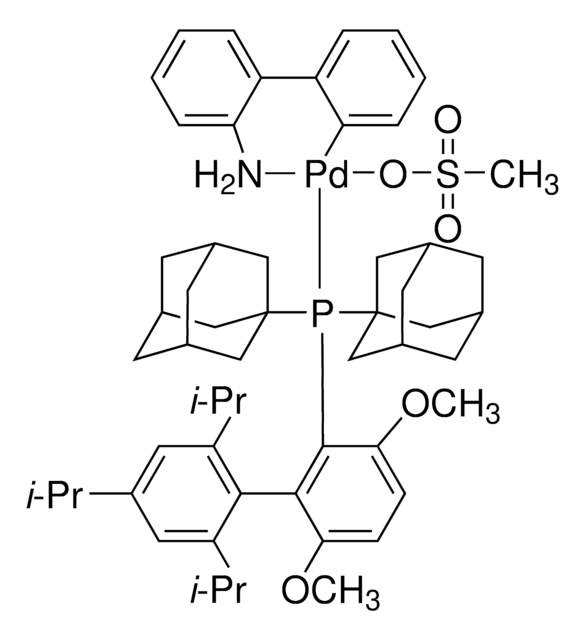
tBuBrettPhos Pd G3
96%
Synonyme(s) :
tert-BuBrettPhos-Pd-G3, [(2-Di-tert-butylphosphino-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
Produits recommandés
Niveau de qualité
Pureté
96%
Forme
solid
Caractéristiques
generation 3
Pertinence de la réaction
core: palladium
reagent type: catalyst
reaction type: Cross Couplings
Capacité de réaction
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
Pf
119-131 °C
Groupe fonctionnel
phosphine
Chaîne SMILES
COC1=CC=C(C(P(C(C)(C)C)C(C)(C)C)=C1C2=C(C=C(C=C2C(C)C)C(C)C)C(C)C)OC.NC3=C(C=CC=C3)C4=C(C=CC=C4)[Pd]OS(C)(=O)=O
InChI
1S/C31H49O2P.C12H10N.CH4O3S.Pd/c1-19(2)22-17-23(20(3)4)27(24(18-22)21(5)6)28-25(32-13)15-16-26(33-14)29(28)34(30(7,8)9)31(10,11)12;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h15-21H,1-14H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
Clé InChI
GAQPAUHHECNHNS-UHFFFAOYSA-M
Description générale
Application
For small scale and high throughput uses, product is also available as ChemBeads (928313)
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Contenu apparenté
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Explore reliable, premium grade catalysis materials for your pharma or industrial project. Specialty chemicals and formulations are available in bulk quantities and volumes from a few grams to multi-metric tons with complete documentation to simplify your leap from development to commercialization.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique