718149
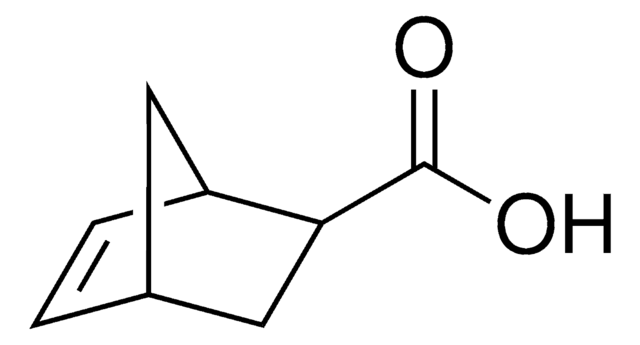
exo-5-Norbornenecarboxylic acid
97%
Synonyme(s) :
(1R,2S,4R)-Bicyclo[2.2.1]hept-5-ene-2-carboxylic acid, NC
About This Item
Produits recommandés
Niveau de qualité
Pureté
97%
Forme
solid
Pf
40-44 °C
Chaîne SMILES
OC(=O)[C@@H]1C[C@@H]2C[C@H]1C=C2
InChI
1S/C8H10O2/c9-8(10)7-4-5-1-2-6(7)3-5/h1-2,5-7H,3-4H2,(H,9,10)/t5-,6+,7+/m0/s1
Clé InChI
FYGUSUBEMUKACF-RRKCRQDMSA-N
Description générale
Application
- A starting material in the synthesis of the metathesis polymer via a ring-opening metathesis polymerization (ROMP) reaction of the ester of exo-5-norbornenecarboxylic acid and 1,1′-bi-2-naphthol.
- A monomer in the preparation of thin films via surface-initiated polymerization process. The resulting thin film serves as a template for selective deposition and etching of metal oxides, which is of significant importance in the microelectronic industry.
- Different crosslinkers for ring-opening metathesis polymerization.
- Norbornene-functionalized monomers, which are used to make poly(norbornene)s via ring-opening metathesis polymerization (ROMP).
- Hydrolytically cleavable and hydrolytically stable functionalized macromonomers for hydrogel preparation.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
>230.0 °F
Point d'éclair (°C)
> 110 °C
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique