522856
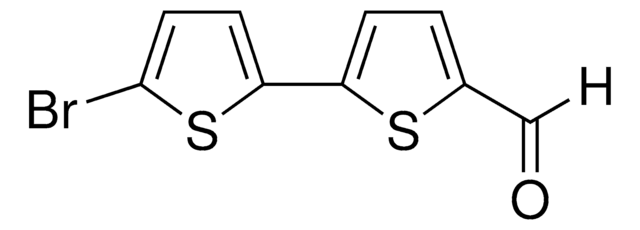
5-Bromo-2,2′-bithiophene
96%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H5BrS2
Número de CAS:
Peso molecular:
245.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
96%
form
solid
mp
29-32 °C (lit.)
SMILES string
Brc1ccc(s1)-c2cccs2
InChI
1S/C8H5BrS2/c9-8-4-3-7(11-8)6-2-1-5-10-6/h1-5H
InChI key
OMOAIGVIYUXYAU-UHFFFAOYSA-N
General description
5-Bromo-2,2′-bithiophene is a bromothiophene derivative. Its reaction with various aryl iodides bearing an electron-donating or electron-withdrawing substituent has been described. It can be synthesized from 2,2′-bithiophene.
Application
5-Bromo-2,2′-bithiophene may be used in the synthesis of trimethyl-[2,2′;5′,2″;5″,2″]quaterthiophen-5-yl-silane (4TTMS) and 5-hexylsulfanyl-2,2′:5′,2′′-terthiophene.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Mark E Roberts et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12134-12139 (2008-08-20)
The development of low-cost, reliable sensors will rely on devices capable of converting an analyte binding event to an easily read electrical signal. Organic thin-film transistors (OTFTs) are ideal for inexpensive, single-use chemical or biological sensors because of their compatibility
Silole-Containing. pi.-Conjugated Systems. 3.1 A Series of Silole-Thiophene Cooligomers and Copolymers: Synthesis, Properties, and Electronic Structures.
Tamao K, et al.
Macromolecules, 28(25), 8668-8675 (1995)
Kei Kobayashi et al.
Organic letters, 7(22), 5083-5085 (2005-10-21)
[reaction: see text] Bromothiophene derivatives react with aryl iodides catalyzed by a palladium complex in the presence of a silver(I) nitrate/potassium fluoride system to induce coupling at the C-H bond, while the carbon-bromine bond is intact. The produced coupling product
Three-dimensional tetra (oligothienyl) silanes as donor material for organic solar cells.
Roquet S, et al.
Journal of Materials Chemistry, 16(29), 3040-3045 (2006)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)