483869
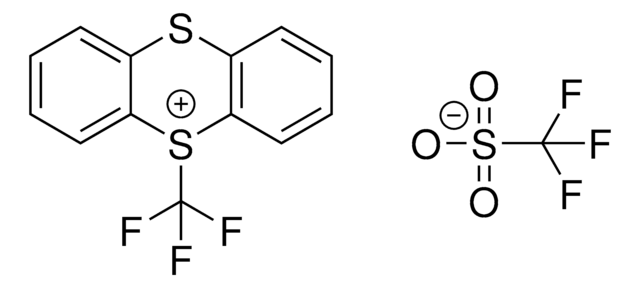
5-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate
97%
Sinónimos:
S-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate, Umemoto reagent
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C13H8BF7S
Número de CAS:
Peso molecular:
340.07
Número MDL:
Código UNSPSC:
12352101
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
97%
mp
162-164 °C (lit.)
grupo funcional
fluoro
cadena SMILES
F[B-](F)(F)F.FC(F)(F)[S+]1c2ccccc2-c3ccccc13
InChI
1S/C13H8F3S.BF4/c14-13(15,16)17-11-7-3-1-5-9(11)10-6-2-4-8-12(10)17;2-1(3,4)5/h1-8H;/q+1;-1
Clave InChI
VTVISWLINKWMQZ-UHFFFAOYSA-N
Aplicación
- Pd(II)-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl boronic acids using a Collidine as a trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes
Used in the stereoselective preparation of
- Trifluoromethyl-substituted alkenes via copper-catalyzed trifluoromethylation of terminal alkenes
- Trifluoromethyl-bearing quaternary carbon centers by Pd-catalyzed intramolecular decarboxylative allylation of α-trifluoromethyl β-keto esters
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Construction of Trifluoromethyl-Bearing Quaternary Carbon Centers by Intramolecular Decarboxylative Allylation of α-Trifluoromethyl β-Keto Esters
Shibata, N.; et al.
Advanced Synthesis & Catalysis, 353, 2037-2041 (2011)
Jun Xu et al.
Chemical communications (Cambridge, England), 47(14), 4300-4302 (2011-03-08)
A copper-catalyzed process for trifluoromethylation of aryl, heteroaryl, and vinyl boronic acids has been developed. The reaction is conducted under mild conditions and shows tolerance to moisture and a variety of functional groups.
Jun Xu et al.
Journal of the American Chemical Society, 133(39), 15300-15303 (2011-09-15)
An unprecedented type of reaction for Cu-catalyzed trifluoromethylation of terminal alkenes is reported. This reaction represents a rare instance of catalytic trifluoromethylation through C(sp(3))-H activation. It also provides a mechanistically unique example of Cu-catalyzed allylic C-H activation/functionalization. Both experimental and
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
Xing-Guo Zhang et al.
Journal of the American Chemical Society, 134(29), 11948-11951 (2012-07-12)
A Pd(II)-catalyzed trifluoromethylation of ortho C-H bonds with an array of N-arylbenzamides derived from benzoic acids is reported. N-Methylformamide has been identified as a crucial promoter of C-CF(3) bond formation from the Pd center. X-ray characterization of the C-H insertion
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico