412562
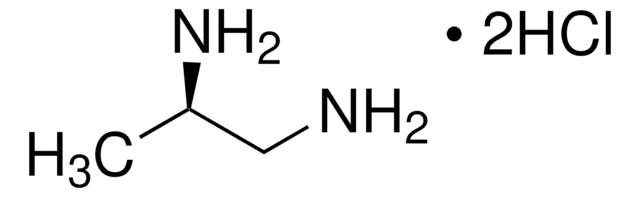
(S)-(−)-1,2-Diaminopropane dihydrochloride
99%
Sinónimos:
(S)-(−)-Propylenediamine dihydrochloride, (S)-1,2-Propanediamine dihydrochloride
About This Item
Productos recomendados
Quality Level
assay
99%
optical activity
[α]22/D −4°, c = 20 in H2O
mp
227-229 °C (lit.)
SMILES string
Cl.Cl.C[C@H](N)CN
InChI
1S/C3H10N2.2ClH/c1-3(5)2-4;;/h3H,2,4-5H2,1H3;2*1H/t3-;;/m0../s1
InChI key
AEIAMRMQKCPGJR-QTNFYWBSSA-N
Application
- (S)-(-)-4-methyl-2-(1-naphthylmethyl)imidazoline hydrochloride
- (S)-(-)-2-benzyl-4-methylimidazoline picrate
- (S )-(-)-2-[(2,6-dichlorophenyl)imino]-4-methylimidazolidine hydrochloride
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico