E6754
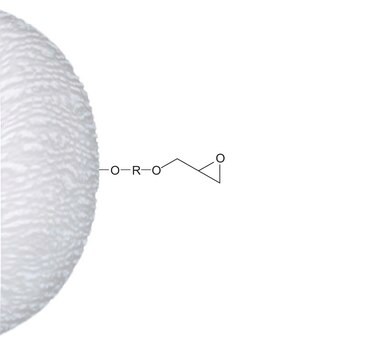
Epoxy-activated−Sepharose™ 6B
lyophilized powder
Synonym(s):
Epoxy-activated-Agarose
About This Item
Recommended Products
form
lyophilized powder
extent of labeling
19-40 μmol per mL
technique(s)
affinity chromatography: suitable
matrix
Sepharose 6B
matrix active group
epoxy
matrix attachment
through epoxy to hydroxyl
matrix spacer
12 atoms
swelling
1 g swells to 3.0-5.0 mL
particle size
45—165 μm
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
Other Notes
Attachment: one epoxy group
Spacer: provides a 12-atom spacer when ligands are coupled to free epoxy groups. Linkage is a stable, uncharged ether.
Physical form
Legal Information
replaced by
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service