391794
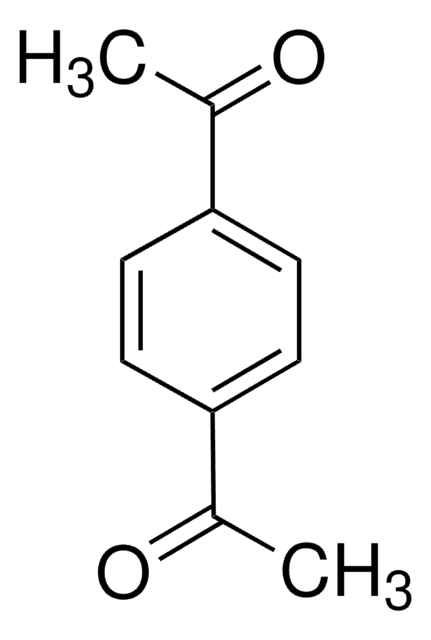
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone
99%
Synonym(s):
4,6-Diacetylresorcinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H2(COCH3)2
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
178-180 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cc(C(C)=O)c(O)cc1O
InChI
1S/C10H10O4/c1-5(11)7-3-8(6(2)12)10(14)4-9(7)13/h3-4,13-14H,1-2H3
InChI key
GEYCQLIOGQPPFM-UHFFFAOYSA-N
General description
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) is a bifunctional carbonyl compound. Its synthesis by acetylating resorcinol in the presence of zinc chloride has been reported. The crystal structure of DAR has been studied.
Application
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) may be used in the synthesis of the following:
- Schiff base ligands
- hexadentate chalcogenated bisimine ligands
- 1,5-benzodiazepines
- ketimine of chitosan
- mannich bases
- hydrazone ligands
- thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands
- binuclear cobalt(II) and copper(II) complexes
- europium (III) complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mohammed Sardaryar Khan et al.
Acta poloniae pharmaceutica, 67(3), 261-266 (2010-06-09)
In the present study, a series of Mannich bases was synthesized by condensing 4,6-diacetylresorcinol with formaldehyde and some selected secondary amines following the Mannich reaction conditions. Findings revealed that Mannich reaction did not take place at the acetyl function but
Magdy Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 113, 356-366 (2013-06-08)
Reactions of 4,6-diacetylresorcinol with different cobalt(II) and copper(II) salts viz., OAc(-), Cl(-), NO3(-) and SO4(2-), yielded a new series of binuclear metal complexes. Reactions of the ligand with these metal ions in the presence of a secondary ligand (L') [O,O-donor;
Design, Synthesis and Characterization of Bimetallic Palladium Complexes for Terminal Olefin Epoxidation
Netalkar SP, et al.
Catalysis Letters, 144(9), 1573-1583 (2014)
Magdy Shebl
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(4), 850-859 (2007-11-13)
A tetradentate N2O2 donor Schiff base ligand, H2L, was synthesized by the condensation of 4,6-diacetylresorcinol with benzylamine. The structure of the ligand was elucidated by elemental analyses, IR, 1H NMR, electronic and mass spectra. Reaction of the Schiff base ligand
Spectral characterization and biological evaluation of Schiff bases and their mixed ligand metal complexes derived from 4, 6-diacetylresorcinol.
Pandya JH, et al.
Journal of Saudi Chemical Society, 18(3), 190-199 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service