H3648
(±)-3-Hydroxydecanoic acid
≥98%
Sinonimo/i:
DL-β-Hydroxycapric acid
About This Item
Prodotti consigliati
Saggio
≥98%
Forma fisica
powder
Gruppo funzionale
carboxylic acid
Tipo di lipide
saturated FAs
Condizioni di spedizione
ambient
Temperatura di conservazione
2-8°C
Stringa SMILE
CCCCCCCC(O)CC(O)=O
InChI
1S/C10H20O3/c1-2-3-4-5-6-7-9(11)8-10(12)13/h9,11H,2-8H2,1H3,(H,12,13)
FYSSBMZUBSBFJL-UHFFFAOYSA-N
Applicazioni
- LORE receptor homomerization is required for 3-hydroxydecanoic acid-induced immune signaling and determines the natural variation of immunosensitivity within the Arabidopsis genus.: This study unveils the crucial role of 3-Hydroxydecanoic acid in mediating immune responses through LORE receptor homomerization in plants, providing insights into the molecular mechanisms of plant defense and potential agricultural applications (Eschrig et al., 2024).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.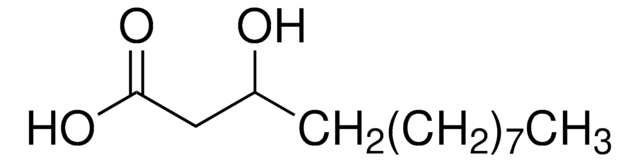


![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)