PHR1669
Lidocaine Related Compound A
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
2,6-Dimethylaniline, Lidocaine Impurity A; 2,6 DMA, 2,6-Xylidine, 2-Amino-1,3-dimethylbenzene, 2-Amino-m-xylene
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to Ph. Eur. Y0001575
Tensione di vapore
<0.01 mmHg ( 20 °C)
Famiglia di API
lidocaine
CdA
current certificate can be downloaded
Confezionamento
pkg of 100 mg
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.560 (lit.)
P. eboll.
214 °C/739 mmHg (lit.)
Punto di fusione
10-12 °C (lit.)
Densità
0.984 g/mL at 25 °C (lit.)
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-30°C
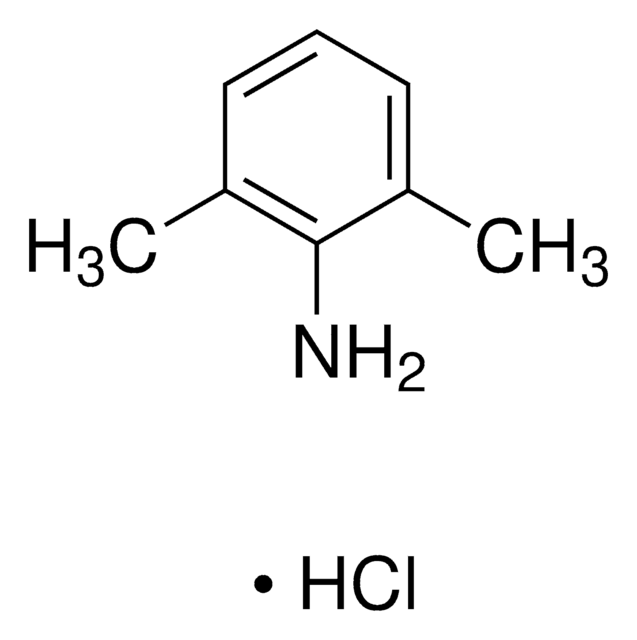
Stringa SMILE
Cc1cccc(C)c1N
InChI
1S/C8H11N/c1-6-4-3-5-7(2)8(6)9/h3-5H,9H2,1-2H3
UFFBMTHBGFGIHF-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
The standard is a certified reference material (CRM) qualified with instruments validated according to good manufacturing practices (GMP) using pharmacopeia monograph methods. It is supplied with a comprehensive certificate containing information on traceability assay results, certified purity, homogeneity tests, uncertainty statement, and stability assessment.
Lidocaine Related Compound A is a primary aromatic amine and a major metabolite of the anesthetic lidocaine. It is used as a starting material in the manufacturing of various anesthetics like lidocaine, bupivacaine, mepivacaine, etidocaine, ropivacaine, pyrrocaine, and xylazine.
Applicazioni
This pharmaceutical secondary standard can also be used as follows:
- Development of an impurity selective reverse phase-high performance liquid chromatography (RP-HPLC) method to determine dexpanthenol, lidocaine hydrochloride, mepyramine maleate, and their related substances in topical dosage forms
- Testing a selective high-performance liquid chromatography-diode array detection (HPLC-DAD) method, developed for the simultaneous analysis of miconazole nitrate and lidocaine hydrochloride in their combined oral gel dosage form, for its stability-indicating properties
- Evaluation of a high-performance liquid chromatography-diode array detection (HPLC-DAD) procedure― for its stability indicating properties, developed to determine nitrofurazone and lidocaine hydrochloride in their combined dosage form
- Separation of 2,6-Dimethylaniline, its isomeric impurities, and other related impurities by isocratic and reverse-phase ultra-performance liquid chromatographic (UPLC) method
- analyze a binary mixture of lidocaine hydrochloride and cetylpyridinium chloride in presence of lidocaine impurity A by spectrophotometric methods
- determine lidocaine hydrochloride-related substance by analytical methods in pharmaceutical dosage forms
Risultati analitici
Nota a piè di pagina
Prodotti consigliati
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
195.8 °F - closed cup
Punto d’infiammabilità (°C)
91 °C - closed cup
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.