PHR1032
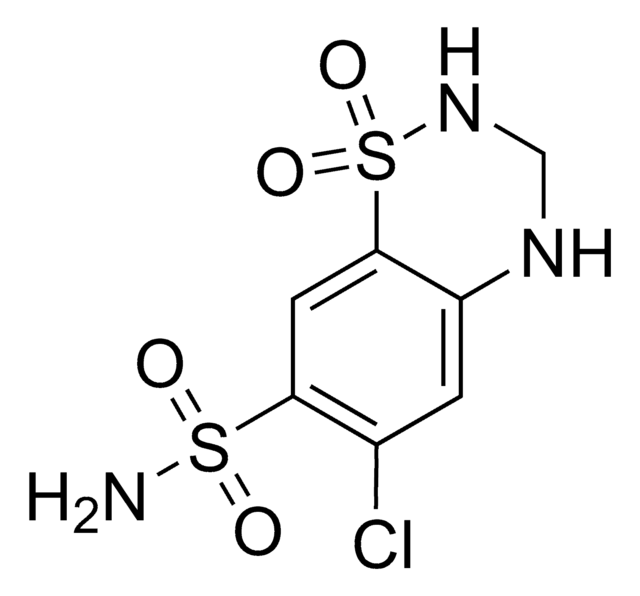
Hydrochlorothiazide
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
HCTZ, 6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide, 6-Chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to BP 186
traceable to Ph. Eur. H1200000
traceable to USP 1314009
Famiglia di API
hydrochlorothiazide
CdA
current certificate can be downloaded
tecniche
HPLC: suitable
gas chromatography (GC): suitable
applicazioni
forensics and toxicology
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-30°C
Stringa SMILE
NS(=O)(=O)c1cc2c(NCNS2(=O)=O)cc1Cl
InChI
1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13)
JZUFKLXOESDKRF-UHFFFAOYSA-N
Informazioni sul gene
human ... SLC12A3(6559)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Hydrochlorothiazide is an antihypertensive and a diuretic that is used for the control of hypertension.
Applicazioni
Azioni biochim/fisiol
Risultati analitici
Altre note
Nota a piè di pagina
Prodotti consigliati
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Carc. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.