H7753
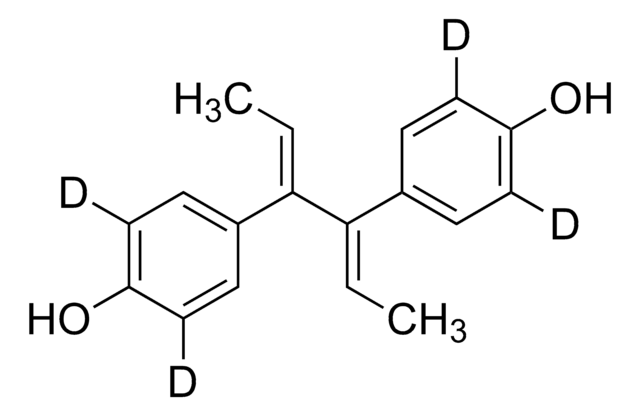
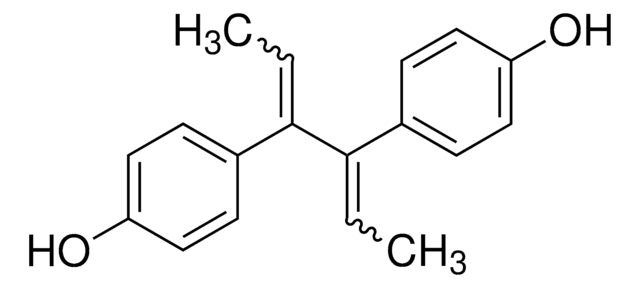
Hexestrol
analytical standard
Sinonimo/i:
4,4′-(1,2-Diethylethylene)diphenol, meso-3,4-Bis(4-hydroxyphenyl)hexane, Dihydrodiethylstilbestrol
About This Item
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
Saggio
≥98%
tecniche
HPLC: suitable
gas chromatography (GC): suitable
applicazioni
forensics and toxicology
pharmaceutical (small molecule)
veterinary
Formato
neat
Stringa SMILE
CC[C@H]([C@H](CC)c1ccc(O)cc1)c2ccc(O)cc2
InChI
1S/C18H22O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,17-20H,3-4H2,1-2H3/t17-,18+
PBBGSZCBWVPOOL-HDICACEKSA-N
Informazioni sul gene
human ... ESR1(2099)
rat ... Esr1(24890)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Linkage
Prodotti consigliati
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Carc. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
DNA damage and repair mechanism is vital for maintaining DNA integrity. Damage to cellular DNA is involved in mutagenesis, the development of cancer among others.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.