It was verry easy to check the items i need and show me the lead time.
11709
Barbituric acid
for spectrophotometric det. of cyanide, ≥99.5%
Sinonimo/i:
2,4,6-Trihydroxypyrimidine, Malonylurea
Scegli un formato
Scegli un formato
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥99.5% (HPLC)
≥99.5%
Stato
solid
Qualità
for spectrophotometric det. of cyanide
tecniche
UV/Vis spectroscopy: suitable
Residuo alla calcinazione
≤0.05%
Punto di fusione
248-252 °C (dec.) (lit.)
Anioni in tracce
chloride (Cl-): ≤50 mg/kg
sulfate (SO42-): ≤500 mg/kg
Cationi in tracce
Ca: ≤10 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
Mg: ≤10 mg/kg
Mn: ≤5 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Zn: ≤5 mg/kg
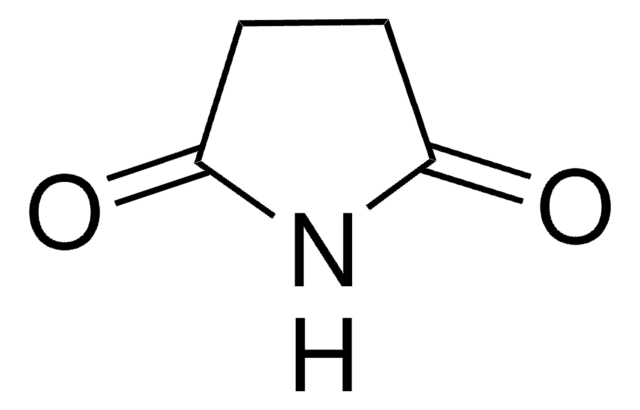
Stringa SMILE
O=C1CC(=O)NC(=O)N1
InChI
1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9)
HNYOPLTXPVRDBG-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
302.0 °F - closed cup
Punto d’infiammabilità (°C)
150.00 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.







![Cyanide standard solution traceable to SRM from NIST K₂[Zn(CN)₄] in H₂O 1000 mg/l CN Certipur®](/deepweb/assets/sigmaaldrich/product/images/920/032/af45eec3-100b-4996-8eb3-c3942d441bc9/640/af45eec3-100b-4996-8eb3-c3942d441bc9.jpg)