03000590
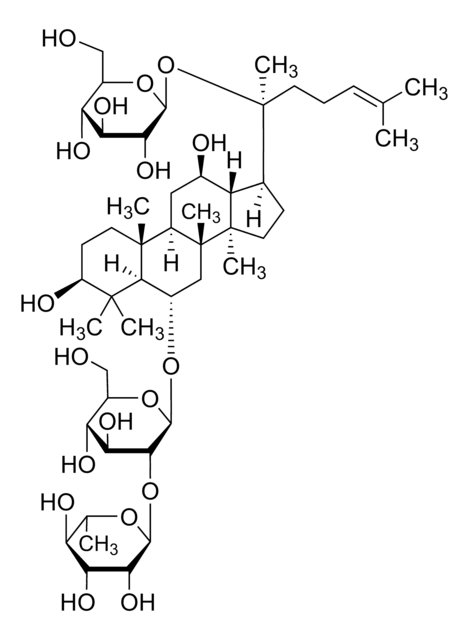
Ginsenoside Re
primary reference standard
Sinonimo/i:
(3β,6α,12β)-20-(β-D-Glucopyranosyloxy)-3,12-dihydroxydammar-24-en-6-yl 2-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside, Chikusetsusaponin IVc, Ginsenoside B2, NSC 308877, Panaxoside Re, Sanchinoside Re
Scegli un formato
About This Item
Prodotti consigliati
Grado
primary reference standard
Durata
limited shelf life, expiry date on the label
Produttore/marchio commerciale
HWI
applicazioni
food and beverages
Stringa SMILE
CC1O[C@@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2O[C@H]3C[C@]4(C)[C@H](C[C@@H](O)[C@@H]5[C@H](CC[C@@]45C)[C@](C)(CC\C=C(/C)C)O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)[C@@]7(C)CC[C@H](O)C(C)(C)[C@H]37)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C48H82O18/c1-21(2)11-10-14-48(9,66-42-38(60)35(57)32(54)26(19-49)63-42)23-12-16-46(7)30(23)24(51)17-28-45(6)15-13-29(52)44(4,5)40(45)25(18-47(28,46)8)62-43-39(36(58)33(55)27(20-50)64-43)65-41-37(59)34(56)31(53)22(3)61-41/h11,22-43,49-60H,10,12-20H2,1-9H3/t22-,23-,24+,25-,26+,27+,28?,29-,30+,31-,32+,33+,34+,35?,36-,37+,38+,39+,40-,41-,42-,43+,45+,46+,47+,48-/m0/s1
PWAOOJDMFUQOKB-QVPADXGFSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Exact content by quantitative NMR can be found on the certificate.
Applicazioni
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Optimize HPLC method for ginsenoside separation using a mixture, applying it to American Ginseng root, with conditions and chromatograms shown.
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.