929336
Pomalidomide-C5-phosphoramidite
Sinonimo/i:
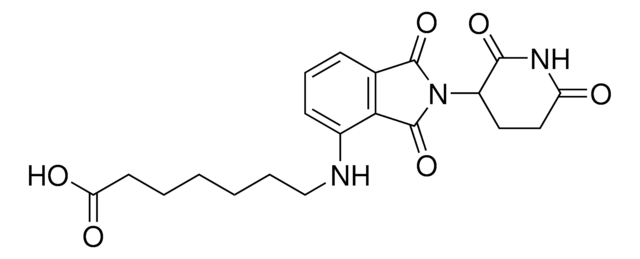
2-Cyanoethyl (5-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)pentyl) diisopropylphosphoramidite
About This Item
Prodotti consigliati
ligand
pomalidomide
Livello qualitativo
Stato
powder
Temperatura di conservazione
2-8°C
Stringa SMILE
O=C1C2=C(C(NCCCCCOP(OCCC#N)N(C(C)C)C(C)C)=CC=C2)C(N1C3C(NC(CC3)=O)=O)=O
InChI
1S/C27H38N5O6P/c1-18(2)32(19(3)4)39(38-17-9-14-28)37-16-7-5-6-15-29-21-11-8-10-20-24(21)27(36)31(26(20)35)22-12-13-23(33)30-25(22)34/h8,10-11,18-19,22,29H,5-7,9,12-13,15-17H2,1-4H3,(H,30,33,34)
LDWPFUQTEOHLBX-UHFFFAOYSA-N
Categorie correlate
Applicazioni
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Altre note
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.