901606
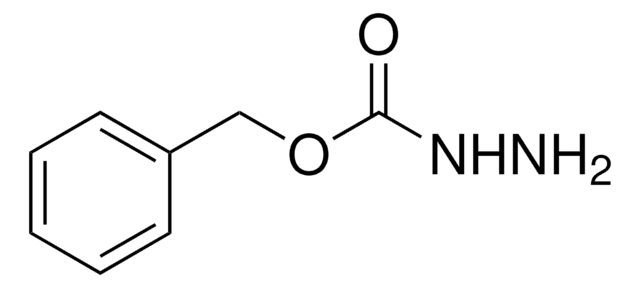
tert-Butyl carbazate solution
1.0 M in dichloromethane
Sinonimo/i:
tert-Butoxycarbonyl hydrazide, tert-Butyl hydrazinecarboxylate, Boc-hydrazide
About This Item
Prodotti consigliati
Stato
liquid
Concentrazione
1.0 M in dichloromethane
Indice di rifrazione
n/D 1.427
Densità
1.290 g/mL
Gruppo funzionale
amine
hydrazine
InChI
1S/C5H12N2O2/c1-5(2,3)9-4(8)7-6/h6H2,1-3H3,(H,7,8)
DKACXUFSLUYRFU-UHFFFAOYSA-N
Applicazioni
Reagent used in solid phase peptide synthesis[2] and in α-amino aldehyde optical purity determinations.[3] Condenses with aldehydes to form hydrazones which are intermediates in the synthesis of HIV-1 protease inhibitors.[4]
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Central nervous system
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.