764639
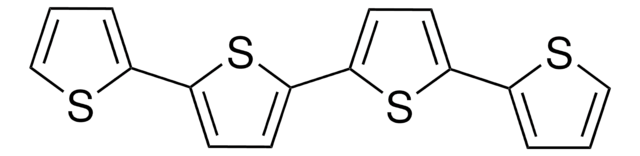
5,5′′′-Bis(tridecafluorohexyl)-2,2′:5′,2 ′′:5′′,2′′′-quaterthiophene
Sinonimo/i:
α,ω-Diperfluorohexyl-quarterthiophene, DFH-4T
About This Item
Prodotti consigliati
Forma fisica
solid
Punto di fusione
205-210 °C
Caratteristiche del semiconduttore
N-type (mobility≤0.64 cm2/V·s)
Stringa SMILE
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)c1ccc(s1)-c2ccc(s2)-c3ccc(s3)-c4ccc(s4)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C28H8F26S4/c29-17(30,19(33,34)21(37,38)23(41,42)25(45,46)27(49,50)51)15-7-5-13(57-15)11-3-1-9(55-11)10-2-4-12(56-10)14-6-8-16(58-14)18(31,32)20(35,36)22(39,40)24(43,44)26(47,48)28(52,53)54/h1-8H
UBMTYFFPSPVBSP-UHFFFAOYSA-N
Descrizione generale
Applicazioni
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.