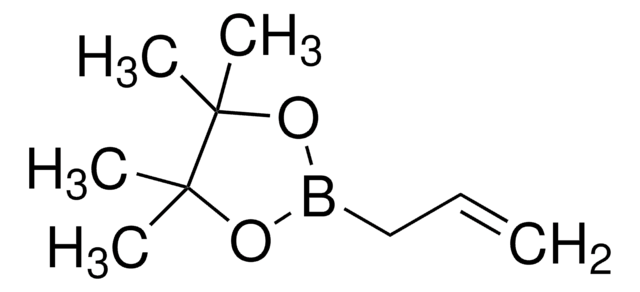
633348
Vinylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
Sinonimo/i:
2-Ethenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 2-Vinyl-4,4,5,5-tetramethyl-1,3,2-dioxaoborolane, 4,4,5,5-Tetramethyl-2-vinyl-1,3,2-dioxaborolane
About This Item
Prodotti consigliati
Saggio
95%
contiene
phenothiazine as stabilizer
Indice di rifrazione
n20/D 1.4300 (lit.)
Densità
0.908 g/mL at 25 °C (lit.)
Temperatura di conservazione
−20°C
Stringa SMILE
CC1(C)OB(OC1(C)C)C=C
InChI
1S/C8H15BO2/c1-6-9-10-7(2,3)8(4,5)11-9/h6H,1H2,2-5H3
DPGSPRJLAZGUBQ-UHFFFAOYSA-N
Applicazioni
- Suzuki-Miyaura coupling reactions
- Mizoroki-Heck reactions (cascade reaction)
- Intramolecular Nozaki-Hiyama-Kishi reactions
- Stereoselective Cu-catalyzed γ-selective and stereospecific coupling
- Control of stereoselectivity and mechanistic portrait on intramolecular (4+1)-cycloaddition of dialkoxycarbenes
- Regio- and stereoselective synthesis of trisubstituted alkenes via gold(I)-catalyzed hydrophosphoryloxylation of haloalkynes followed by Pd-catalyzed consecutive cross-coupling reactions
- Asymmetric Birch reductive alkylation
Reagent used in Preparation of
- Molecular tubes for lipid sensing
- Enzymatic inhibitors, antibiotics, receptor analogs, and other biologically significant compounds (including total syntheses)
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 2 - Flam. Liq. 3 - Skin Sens. 1
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
93.2 °F
Punto d’infiammabilità (°C)
34 °C
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)