556971
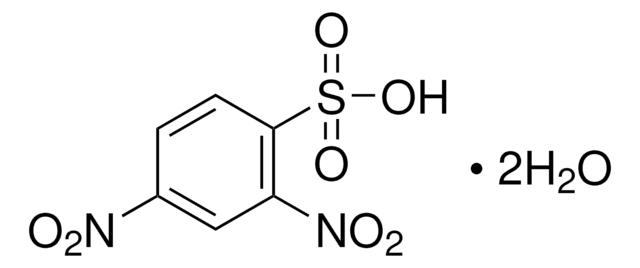
2,4-Dinitrobenzenesulfonic acid hydrate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
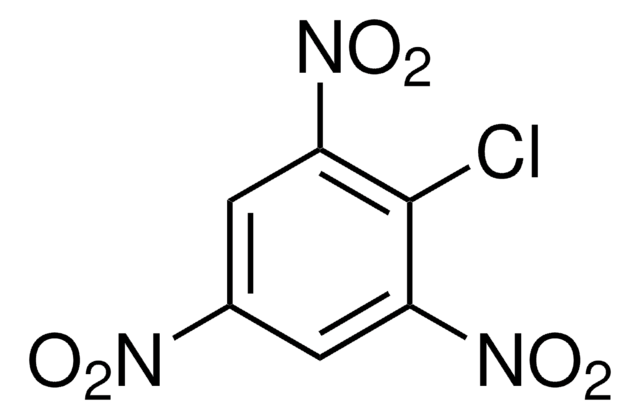
(O2N)2C6H3SO3H · xH2O
Numero CAS:
Peso molecolare:
248.17 (anhydrous basis)
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Punto di fusione
95-98 °C (lit.)
Stringa SMILE
OS(=O)(=O)c1ccc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C6H4N2O7S/c9-7(10)4-1-2-6(16(13,14)15)5(3-4)8(11)12/h1-3H,(H,13,14,15)
OVOJUAKDTOOXRF-UHFFFAOYSA-N
Applicazioni
2,4-Dinitrobenzenesulfonic acid hydrate may be used in the synthesis of the corresponding α-hydroxyketone. It can be used as a catalyst in the amidation reaction, which involves the conversion of secondary-benzylic alcohols to N-benzylacetamides. It may also be used to prepare 1-octyl-2-pyrrolidinonium 2,4-dinitrobenzenesulfonate ([NOP][DNBSA]), a Brønsted-acidic ionic liquid, which can catalyze the synthesis of trioxane.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
"The Ritter reaction under truly catalytic Bronsted acid conditions"
Sanz R, et al.
European Journal of Organic Chemistry, 2007(08), 4642-4645 (2007)
"Bronsted-Acidic Ionic Liquids as Catalysts for Synthesizing Trioxane"
Zhao Y, et al.
Chinese Journal of Chemical Engineering (2016)
Shurong Ren et al.
International immunopharmacology, 72, 284-291 (2019-04-21)
Imiquimod (Imiq) is a synthetic imizoquinoline compound which can act on Toll-like receptor (TLR)7 and transduce signals involved in cell activation. We investigated the role of Imiq on contact hypersensitivity (CHS) and explored the potential mechanisms of mast cells involved
Yejin Yang et al.
Drug design, development and therapy, 13, 231-242 (2019-01-16)
We examined whether metoclopramide (MCP), a modulator of dopamine and serotonin receptors, alleviated colitis and had synergistic effects when coadministered with 5-aminosalicylic acid (5-ASA) in an experimental model of colitis. MCP azo-linked to 5-ASA (5-[4-chloro-2-{2-(diethylamino)ethylcarbamoyl}- 1-methoxyphenyl]azosalicylic acid, MCP-azo-ASA) was synthesized
Tatsuya Ogawa et al.
Journal of immunology (Baltimore, Md. : 1950), 205(4), 907-914 (2020-07-22)
Atopic dermatitis is a chronic form of allergic contact dermatitis that is closely associated with a compromised epidermal barrier. Immunogenicity of a given electrophilic hapten after penetration of this barrier depends directly on biochemical reactions in the thiol-rich layer in
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.