513997
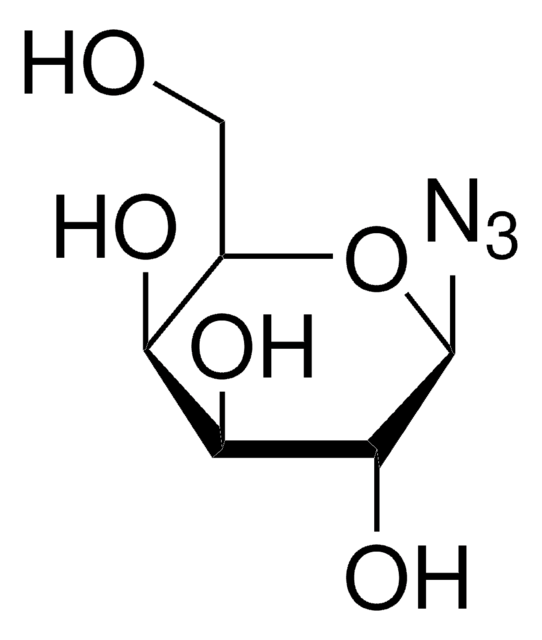
1-Azido-1-deoxy-β-D-glucopyranoside tetraacetate
Sinonimo/i:
NSC 272456
About This Item
Prodotti consigliati
Forma fisica
solid
Attività ottica
[α]/D -29°, c = 1% in H2O
[α]/D -30°, c = 1% in chloroform
Impiego in reazioni chimiche
reaction type: click chemistry
Punto di fusione
127-131 °C (lit.)
Stringa SMILE
CC(=O)OC[C@H]1O[C@@H](N=[N+]=[N-])[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H19N3O9/c1-6(18)22-5-10-11(23-7(2)19)12(24-8(3)20)13(25-9(4)21)14(26-10)16-17-15/h10-14H,5H2,1-4H3/t10-,11+,12+,13-,14-/m1/s1
NHNYHKRWHCWHAJ-MBJXGIAVSA-N
Applicazioni
- 1,2,3-Triazole-boron dipyrromethenes (BODIPYs) containing glucose groups via Cu(I)-catalyzed azide–alkyne ″click″ cycloaddition reaction conditions.
- 1-(β-D-glycosyl)-5-benzenesulfonamide-1,2,3-triazole derivatives by ruthenium-catalyzed azide-alkyne cycloaddition reactions.
- 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosylamine by palladium catalyzed hydrogenation reaction.
- Glycoside annulated dihydropyrimidinone derivatives by one-pot five-component condensation reaction with tert-butyl β-ketoester, arylaldehyde, urea and propargyl alcohol.
- Synthesis of Protein Tyrosine Phosphatase 1B inhibitor
- Synthesis of glycoconjugate carbonic anhydrase inhibitors by ruthenium-catalyzed azide-alkyne 1,3-dipolar cycloaddition
- Synthesis of variously coupled conjugates of D-glucose via click chemistry for inhibition of glycogen phosphorylase
- Hydrogenation reactions
- Preparation of posttranslationally modified peptides efficiently mimicking neoantigens in relation to autoimmune disease
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.