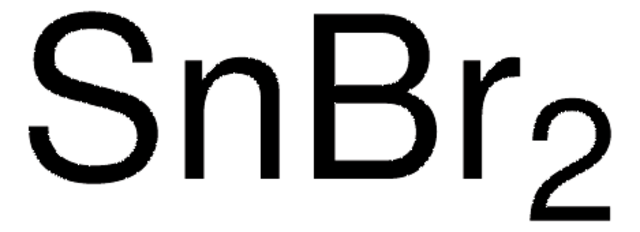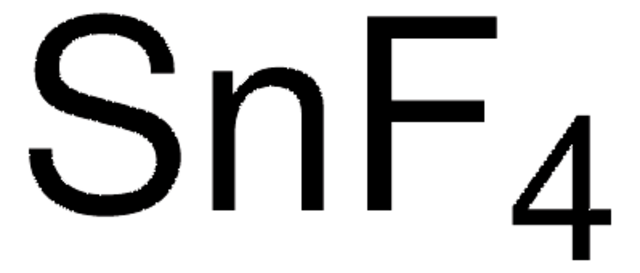The method of preparation for these products is considered proprietary; however, both items are manufactured from the same synthesized precursor. Each item is prepared using a unique method to meet specifications, i.e., powder or beaded form, purity. Neither are additionally purified.
466352
Tin(II) iodide
−10 mesh, 99.999% trace metals basis
Sinonimo/i:
Stannous iodide, Tin diiodide
Scegli un formato
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
99.999% trace metals basis
Stato
powder
Impurezze
≤15.0 ppm Trace Metal Analysis
P. ebollizione
714 °C (lit.)
Punto di fusione
320 °C (lit.)
Densità
5.28 g/mL at 25 °C (lit.)
Stringa SMILE
I[SnH2]I
InChI
1S/2HI.Sn/h2*1H;/q;;+2/p-2
JTDNNCYXCFHBGG-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- As a precursor to fabricate methylammonium tin iodide films for heterojunction-depleted perovskite solar cells using solvent-mediated crystallization.[1] [1]
- To prepare highly stable perovskite thin films for Pb-free light-emitting diodes(PeLEDs). [2] and a new air-stable one-dimensional (1D) hybrid lead-free halide material (DAO)Sn2I6 which emits broad orange light at 634 nm and photoluminescence quantum yield of 36%.[3]
- As a dual-functional electrolyte additive in high-rate Li-air batteries. SnI2 protects the lithium anode and reduces charge potential by promoting the decomposition of the Li2O2.[4]
- To synthesize mixed Pb–Sn perovskite films with formamidine sulfinic acid(FSA) for all tandem perovskite solar cells.[5]
Accessorio
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 - STOT SE 3
Organi bersaglio
Cardio-vascular system,hematopoietic system, Respiratory system
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
-
This is important to whether I buy your product.I would like to ask whether 409308 and 466352 are products obtained by two different synthetic routes or 466352 was obtained by further purifying 409308. And how do you synthesize and purify Tin(II) iodide?
1 answer-
Helpful?
-
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.