433985
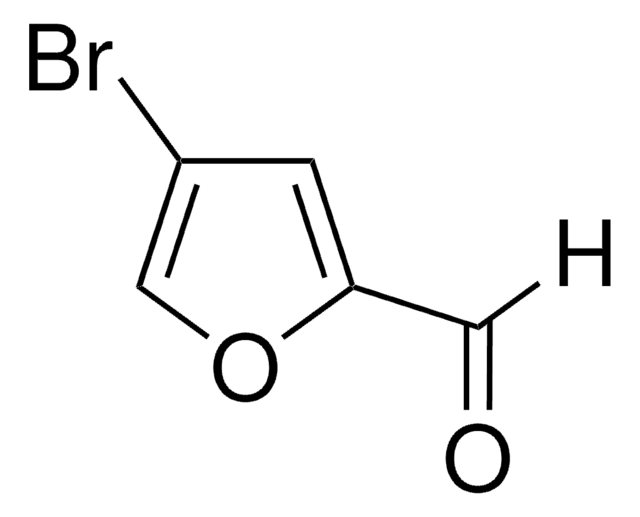
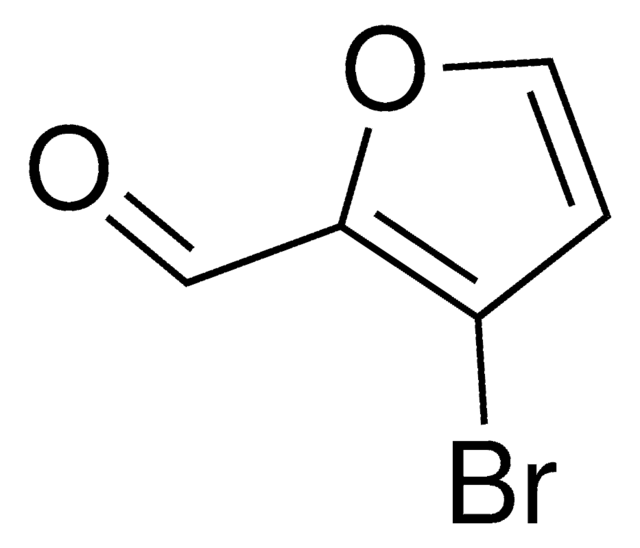
5-Bromo-2-furaldehyde
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H3BrO2
Numero CAS:
Peso molecolare:
174.98
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
P. ebollizione
112 °C/16 mmHg (lit.)
Punto di fusione
82-85 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
Brc1ccc(C=O)o1
InChI
1S/C5H3BrO2/c6-5-2-1-4(3-7)8-5/h1-3H
WJTFHWXMITZNHS-UHFFFAOYSA-N
Applicazioni
5-Bromo-2-furaldehyde may be employed for the following syntheses:
- 5-substituted 2-furaldehydes
- anilines, through a novel one-pot, two-step amination/Diels-Alder procedure
- 5-(5′,8′-dimethyl-9′-tert-butoxycarbonyl-9′H-carbazol-3′-yl)-furan-2-carbaldehyde
- 5-(6-hydroxyhexyl)-2-furaldehyde
- 5-methylsulfonyl-2-furaldehyde
- 5-phenylsulfonyl-2-furaldehyde
- 5-(4-acetamidobenzylsulfonyl)-2-furaldehyde
- 5-iodo-2-furaldehyde
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Aminomethylations via cross-coupling of potassium organotrifluoroborates with aryl bromides.
Molander GA and Sandrock DL.
Organic Letters, 9(8), 1597-1600 (2007)
Raouf Medimagh et al.
The Journal of organic chemistry, 73(6), 2191-2197 (2008-02-28)
Selective metal-free amination and Diels-Alder reactions are described in the furan series, leading to polysubstituted anilines or to stable oxabicyclic adducts in high yield. Interestingly, anilines are conveniently prepared through a novel one-pot, two-step amination/Diels-Alder procedure from commercially available 5-bromo-2-furaldehyde.
Furan derivatives. LXXXV11. The synthesis and ultraviolet spectra of 5-(4-X-phenyIsulfonyI)-2-furaldehydes and 2-cyano-3-[5-(4-X-phenyl-sulfonyl)-2-furyl] acrylonitriles.
Kada R and Kovac J.
Chemical Papers, 30(4), 502-507 (1976)
Gary A Molander et al.
Organic letters, 9(8), 1597-1600 (2007-03-21)
[reaction: see text] The Suzuki-Miyaura cross-coupling reaction of N,N-dialkylaminomethyltrifluoroborates with aryl halides allows the construction of an aminomethyl aryl linkage through a disconnection based on dissonant reactivity patterns. A variety of these aminomethyltrifluoroborate substrates were prepared in good to excellent
Photochemical coupling between halogenoheterocyclic and heterocyclic derivatives.
D'Agostini A and D'Auria M.
Journal of the Chemical Society. Perkin Transactions 1, 9, 1245-1249 (1994)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.