423807
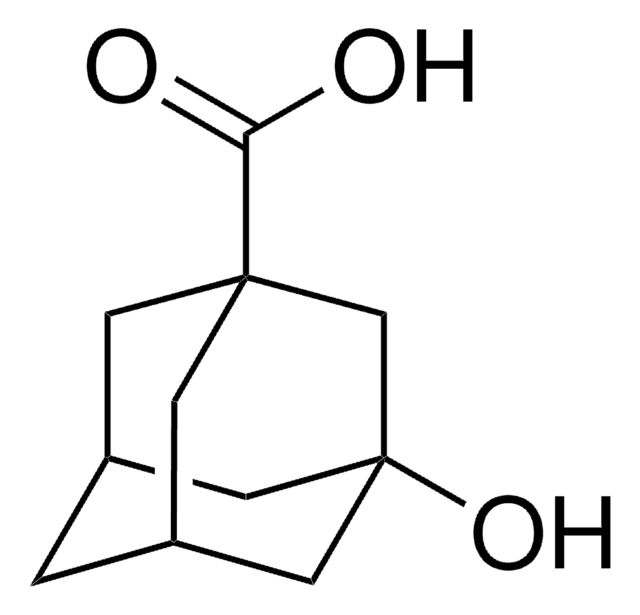
5-Hydroxy-2-adamantanone
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H14O2
Numero CAS:
Peso molecolare:
166.22
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
solid
Punto di fusione
>300 °C (lit.)
Stringa SMILE
O[C@]12C[C@@H]3C[C@H](C1)C(=O)[C@@H](C3)C2
InChI
1S/C10H14O2/c11-9-7-1-6-2-8(9)5-10(12,3-6)4-7/h6-8,12H,1-5H2/t6-,7-,8+,10-
TZBDEVBNMSLVKT-XYYXLIQBSA-N
Descrizione generale
5-Hydroxy-2-adamantanone is a disubstituted derivative of adamantane. The biocatalyzed synthesis of 5-hydroxy-2-adamantanone from 2-adamantanone has been investigated.
Applicazioni
5-Hydroxy-2-adamantanone may be used in the following studies:
- As a model compound to investigate the application of lanthanide NMR shift reagents for the analysis of disubstituted derivative of adamantane.
- As a starting material for the synthesis of E-2-amino-5-hydroxyadamantane.
- As a starting material for the synthesis of 4-(triphenylsilyloxy)adamantan-1-ol.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
[The immunomodulator kemantan in the treatment of patients with exacerbated chronic obstructive bronchitis].
E M Rekalova
Likars'ka sprava, (4)(4), 73-76 (1992-04-01)
S S Boĭko et al.
Farmakologiia i toksikologiia, 54(1), 57-59 (1991-01-01)
The pharmacokinetics of a new Soviet-made immunostimulant kemantane, a derivative of adamantine, was studied by gas-liquid chromatography in patients with bronchial pathology. It was found that in the blood of the patients kemantane was not practically detected due to a
An expeditious preparation of E-2-amino-5-hydroxyadamantane and its Z-isomer.
Jaroskova L, et al.
Tetrahedron Letters, 47(46), 8063-8067 (2006)
Marta L Lage et al.
Tetrahedron, 69(27-28), 5609-5613 (2013-09-03)
A chemoselective method for the hydrosilylation of ketones has been developed, using the combination of triphenylsilane and a catalyst prepared from Ni(COD)
Application of shift reagents in the study of disubstituted derivatives of adamantane by NMR spectroscopy.
Vodicka L, et al.
Collection of Czechoslovak Chemical Communications, 40(1), 293-299 (1975)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.