144029
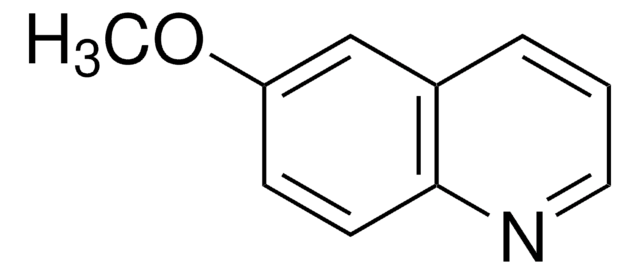
2,6-Dimethylquinoline
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H11N
Numero CAS:
Peso molecolare:
157.21
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
solid
Punto di fusione
57-59 °C (lit.)
Stringa SMILE
Cc1ccc2nc(C)ccc2c1
InChI
1S/C11H11N/c1-8-3-6-11-10(7-8)5-4-9(2)12-11/h3-7H,1-2H3
JJPSZKIOGBRMHK-UHFFFAOYSA-N
Informazioni sul gene
human ... CYP1A2(1544)
Applicazioni
2,6-Dimethylquinoline was used to study the inhibition potencies (IC50 values) of structurally diverse chemicals with recombinant human CYP2B6 enzyme for in vitro research purposes.
Azioni biochim/fisiol
2,6-Dimethylquinoline is the chemical constituent present in roots of Peucedantu praeruptorum. It is a potential inhibitor of cytochrome P450 1A2 activity.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Cun Zhang et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 31(16), 1333-1335 (2006-10-26)
To isolate and identify the chemical constituents from the roots of Peucedantu praeruptorum. The constituents were isolated by column chromatography on silica gel and ODS, and identified by NMR, MS spectroscopic methods. Eight compounds, (-) sclerodin (1), palmitic acid (2)
L E Korhonen et al.
British journal of pharmacology, 150(7), 932-942 (2007-02-28)
The cytochrome P450 2B6 (CYP2B6) enzyme metabolises a number of clinically important drugs. Drug-drug interactions resulting from inhibition or induction of CYP2B6 activity may cause serious adverse effects. The aims of this study were to construct a three-dimensional structure-activity relationship
Bin Yang et al.
Environmental science and pollution research international, 23(4), 3399-3405 (2015-10-23)
The solubilities of 19 different kinds of N-heteroaromatic compounds in aqueous solutions with different concentrations of NaCl were determined at 298.15 K with a UV-vis spectrophotometry and titration method, respectively. Setschenow constants, Ks, were employed to describe the solubility behavior
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Laura E Korhonen et al.
Journal of medicinal chemistry, 48(11), 3808-3815 (2005-05-27)
The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors and to use this model to predict the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.